Chemistry:Trinitroanisole
| |
| Names | |
|---|---|
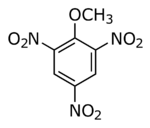
| Preferred IUPAC name
2-Methoxy-1,3,5-trinitrobenzene | |
| Other names
2,4,6-Trinitroanisol; picric acid methyl esther; trisol; trinol; trinitroanisole
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H5N3O7 | |
| Molar mass | 243.131 g·mol−1 |
| Appearance | yellow, "leaf-like" crystals |
| Density | 1.61 g/cm3 |
| Melting point | 68 °C (154 °F; 341 K) |
| Boiling point | explodes |
| insoluble in water, soluble in diethyl ether and hot ethanol | |
| Hazards | |
| Main hazards | explosive |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H201, H302, H312, H332, H411 | |
| P210, P230, P240, P250, P261, P264, P270, P271, P273, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P370+380, P372, P373, P391, P401, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trinitroanisole is a chemical compound that exists as pale yellow crystals with a melting point of 68 °C. It is highly toxic. It is an explosive with a detonation velocity of 7200 meters per second.[1] The compound's primary hazard is a blast of an instantaneous explosion, not flying projectiles or fragments.[2]
Synthesis
Trinitroanisole was first prepared in 1849 by the French chemist Auguste Cahours by reacting p-anisic acid (French: acide anisique) with a mixture of sulfuric acid and fuming nitric acid.[3][4]
Trinitroanisole can be prepared by the reaction of 2,4-dinitrochlorobenzene with methanol in the presence of sodium hydroxide followed by the nitration of the resulting product. Alternatively, it can be prepared directly by the reaction of picryl chloride with methanol in the presence of sodium hydroxide.[1]
Molecular Formula
C7H5N3O7
Synonyms
- 2-Methoxy-1,3,5-trinitrobenzene
- Methyl picrate
- 2,4,6-TRINITROANISOLE
- 606-35-9
- Ether, methyl picryl[2]
Use
Historically, trinitroanisole was used as a military explosive (e.g., Japanese Type 91), however, due to its tendency to form picric acid and dangerous picrate salts, its use has largely been abandoned.
Notes
- ↑ 1.0 1.1 Wasag-Chemie, Essen. "Explosivstoffe". 1961, p. 164.
- ↑ 2.0 2.1 PubChem. "2,4,6-Trinitroanisole" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/11817.
- ↑ Cahours, Auguste (1849). "Researches relatives à l'action du mélange d'acide sulfurique et d'acide nitrique fumants sur les matières organiques" (in French). Annales de Chimie et de Physique. 3rd series 25: 5–44. https://babel.hathitrust.org/cgi/pt?id=hvd.hx3dy4&view=1up&seq=11. See especially pp. 21-30.
- ↑ Fedoroff, Basil T. (1960). Encyclopedia of Explosives and Related Items. 1. Dover, N.J.: Picatinny Arsenal. pp. A450–A453. https://archive.org/stream/Ullmans/01%20Ullmans-U-S-ARMY-Encyclopedia-of-Explosives-and-Related-Items-Vol-01#page/n557/mode/2up/.
 |

