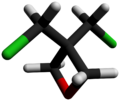
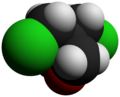
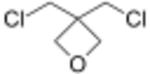
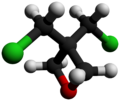
Chemistry:3,3-Bis(chloromethyl)oxetane
From HandWiki
| |||
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
3,3-Bis(chloromethyl)oxetane | |||
| Other names
3,3-Dichloromethyloxycyclobutane
BCMO | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C5H8Cl2O | |||
| Molar mass | 155.02 g·mol−1 | ||
| Appearance | Black or olive green solid[1] | ||
| Density | 1.295 g/cm3 | ||
| Melting point | 18.9 °C (66.0 °F; 292.0 K)[1] | ||
| Boiling point | 95 °C (203 °F; 368 K) | ||
| Hazards | |||
| GHS pictograms |  
| ||
| GHS Signal word | Warning | ||
| H302, H330, H315, H319, H335 | |||
| P260, P264, P270, P271, P280, P284, P301+312, P302+352, P304+340, P305+351+338, P310, P320, P321, P330, P332+313, P337+313, P362+364Script error: No such module "Preview warning".Category:GHS errors, P403+233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
3,3-Bis(chloromethyl)oxetane (BCMO) is an intermediate in the synthesis of poly(bis(azidomethyl)oxetane (PolyBAMO), an energetic polymer that is being studied for use as a propellant binder for rocket fuel.[2]
It is classified as an extremely hazardous substance in the United States .[3] It can cause kidney damage, lacrimation, and somnolence if consumed.[1]
Preparation and reaction
BCMO is formed in solution via cyclization of pentaerythritol trichlorohydrin with a non-organic base like sodium hydroxide.[4]
BAMO can be formed from a reaction of BCMO with sodium azide. This reaction takes place in an alkaline solution with tetrabutyl ammonium bromide, which acts as a phrase transfer catalyst.[4]
References
- ↑ 1.0 1.1 1.2 Oxetane, 3,3-bis(chloromethyl)- at hazmap.nlm.nih.gov.
- ↑ [2]http://www.dtic.mil/dtic/tr/fulltext/u2/a377866.pdf
- ↑ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities". Government Printing Office. http://edocket.access.gpo.gov/cfr_2008/julqtr/pdf/40cfr355AppA.pdf.
- ↑ 4.0 4.1 Pisharath, Sreekumar; Ang, How Ghee (2007). "Synthesis and thermal decomposition of GAP–Poly(BAMO) copolymer" (in en). Polymer Degradation and Stability 92 (7): 1365–1377. doi:10.1016/j.polymdegradstab.2007.03.016.
 |