Chemistry:Sodium azide
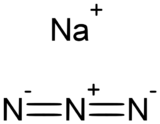
| |
| File:NaN3SmallSection.tif | |

| |
| Names | |
|---|---|
| IUPAC name
Sodium azide
| |
| Other names
Sodium trinitride
Smite Azium | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1687 |
| |
| |
| Properties | |
| NaN 3 | |
| Molar mass | 65.0099 g/mol |
| Appearance | Colorless to white solid |
| Odor | Odorless |
| Density | 1.846 g/cm3 (20 °C) |
| Melting point | 275 °C (527 °F; 548 K) violent decomposition |
| 38.9 g/100 mL (0 °C) 40.8 g/100 mL (20 °C) 55.3 g/100 mL (100 °C) | |
| Solubility | Very soluble in ammonia Slightly soluble in benzene Insoluble in diethyl ether, acetone, hexane, chloroform |
| Solubility in methanol | 2.48 g/100 mL (25 °C) |
| Solubility in ethanol | 0.22 g/100 mL (0 °C) |
| Acidity (pKa) | 4.8 |
| Structure | |
| Hexagonal, hR12[1] | |
| R-3m, No. 166 | |
| Thermochemistry | |
Heat capacity (C)
|
76.6 J/mol·K |
Std molar
entropy (S |
70.5 J/mol·K |
Std enthalpy of
formation (ΔfH⦵298) |
21.3 kJ/mol |
Gibbs free energy (ΔfG˚)
|
99.4 kJ/mol |
| Hazards | |
| Safety data sheet | ICSC 0950 |
| GHS pictograms |    
|
| GHS Signal word | DANGER |
| H300, H310, H410 | |
| P260, P280, P301+310, P501 [2] | |
| NFPA 704 (fire diamond) | |
| Flash point | 300 °C (572 °F; 573 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
27 mg/kg (oral, rats/mice)[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
None[3] |
REL (Recommended)
|
C 0.1 ppm (as HN 3) [skin] C 0.3 mg/m3 (as NaN 3) [skin][3] |
IDLH (Immediate danger)
|
N.D.[3] |
| Related compounds | |
Other anions
|
Sodium cyanide |
Other cations
|
Potassium azide Ammonium azide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium azide is an inorganic compound with the formula NaN
3. This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is highly soluble in water and is acutely poisonous.[5]
Structure
Sodium azide is an ionic solid. Two crystalline forms are known, rhombohedral and hexagonal.[1][6] Both adopt layered structures. The azide anion is very similar in each form, being centrosymmetric with N–N distances of 1.18 Å. The Na+
ion has an octahedral geometry. Each azide is linked to six Na+
centers, with three Na–N bonds to each terminal nitrogen center.[7]
Preparation
The common synthesis method is the "Wislicenus process", which proceeds in two steps in liquid ammonia. In the first step, ammonia is converted to sodium amide by metallic sodium:
- 2 Na + 2 NH
3 → 2 NaNH
2 + H
2
The sodium amide is subsequently combined with nitrous oxide:
- 2 NaNH
2 + N
2O → NaN
3 + NaOH + NH
3
These reactions are the basis of the industrial route, which produced about 250 tons per year in 2004, with production increasing due to the increased use of airbags.[5]
Laboratory methods
Curtius and Thiele developed another production process, where a nitrite ester is converted to sodium azide using hydrazine. This method is suited for laboratory preparation of sodium azide:
- 2 NaNO
2 + 2 C
2H
5OH + H
2SO
4 → 2 C
2H
5ONO + Na
2SO
4 + 2 H
2O - C
2H
5ONO + N
2H
4 · H2O + NaOH → NaN
3 + C
2H
5OH + 3 H
2O
Alternatively the salt can be obtained by the reaction of sodium nitrate with sodium amide.[8]
- 3 NaNH
2 + NaNO
3 → NaN
3 + 3 NaOH + NH
3
Chemical reactions
Acid formation of hydrazoic acid
Treatment of sodium azide with strong acids gives hydrazoic acid (hydrogen azide; HN3):
- H+
+ N−
3 → HN
3
Hydrazoic acid, also extremely toxic, is especially dangerous because it is a gas. Otherwise, aqueous solutions contain only minute amounts of hydrazoic acid, as described by the following equilibrium:
- N−
3 + H
2O ⇌ HN
3 + OH−
, K = 10−4.6
Destruction
Sodium azide can be destroyed by treatment with nitrous acid (HNO
2) generated in situ from a solution of NaN
3 with a metal nitrite by acidification with a mineral acid.[9][10]
- 2 NaN
3 + 2 HNO
2 → 3 N
2 + 2 NO + 2 NaOH
A safer modification to the above method that avoids the potential production of hydrazoic acid or nitrogen oxide fumes is that of W. F. Rinkenbach. A solution of 2.5 oz (71 g) sodium nitrite in 1 US pt (470 mL) of water is added to a stirring dispersion of 1 oz (28 g) sodium azide in 1.5 US gal (5.7 L) 10% ammonium acetate, followed by addition of 7 US fl oz (210 mL) of glacial acetic acid. The solution is allowed to stand in a warm place for an hour and disposed of.[11]
Applications
Automobile airbags and aircraft evacuation slides
Older airbag formulations contained mixtures of oxidizers, sodium azide and other agents including ignitors and accelerants. An electronic controller detonates this mixture during an automobile crash:
- 2 NaN
3 → 2 Na + 3 N
2
The same reaction occurs upon heating the salt to approximately 300 °C. The sodium that is formed is a potential hazard alone and, in automobile airbags, it is converted by reaction with other ingredients, such as potassium nitrate and silica. In the latter case, innocuous sodium silicates are generated.[12] While sodium azide is still used in evacuation slides on modern aircraft, newer-generation automotive air bags contain less sensitive explosives such as nitroguanidine or guanidine nitrate.[13]
Organic and inorganic synthesis
Due to its explosion hazard, sodium azide is of only limited value in industrial-scale organic synthesis. In the laboratory, it is used to introduce the azide functional group by displacement of halides.[10] The azide functional group can thereafter be converted to an amine by reduction with either SnCl
2 in ethanol or lithium aluminium hydride or a tertiary phosphine, such as triphenylphosphine in the Staudinger reaction, with Raney nickel or with hydrogen sulfide in pyridine. Oseltamivir, an antiviral medication, is currently produced in commercial scale by a method which utilizes sodium azide.[14]
Sodium azide is a versatile precursor to other inorganic azide compounds, e.g., lead azide and silver azide, which are used in detonators as primary explosives. These azides are significantly more sensitive to premature detonation than sodium azide and thus have limited applications. Lead and silver azide can be made via double displacement reaction with sodium azide and their respective nitrate (most commonly) or acetate salts. Sodium azide can also react with the chloride salts of certain alkaline earth metals in aqueous solution, such as barium chloride or strontium chloride to respectively produce barium azide and strontium azide, which are also relatively sensitive primarily explosive materials. These azides can be recovered from solution through careful desiccation.
Biochemistry and biomedical uses
Sodium azide is a useful probe reagent, and an antibacterial preservative for biochemical solutions. In the past merthiolate and chlorobutanol were also used as an alternative to azide for preservation of biochemical solutions.[15]
Sodium azide is an instantaneous inhibitor of lactoperoxidase, which can be useful to stop lactroperoxidase catalyzed 125I protein radiolabeling experiments.[16]
In hospitals and laboratories, it is a biocide; it is especially important in bulk reagents and stock solutions which may otherwise support bacterial growth where the sodium azide acts as a bacteriostatic by inhibiting cytochrome oxidase in gram-negative bacteria; however, some gram-positive bacteria (streptococci, pneumococci, lactobacilli) are intrinsically resistant.[17]
Agricultural uses
It is used in agriculture for pest control of soil-borne pathogens such as Meloidogyne incognita or Helicotylenchus dihystera.[18]
It is also used as a mutagen for crop selection of plants such as rice,[19] barley[20] or oats.[21]
Safety considerations
Sodium azide can be fatally toxic,[22] and even minute amounts can cause symptoms. The toxicity of this compound is comparable to that of soluble alkali cyanides,[23] although no toxicity has been reported from spent airbags.[24]
It produces extrapyramidal symptoms with necrosis of the cerebral cortex, cerebellum, and basal ganglia. Toxicity may also include hypotension,[25] blindness and hepatic necrosis. Sodium azide increases cyclic GMP levels in the brain and liver by activation of guanylate cyclase.[26]
Sodium azide solutions react with metallic ions to precipitate metal azides, which can be shock sensitive and explosive. This should be considered for choosing a non-metallic transport container for sodium azide solutions in the laboratory. This can also create potentially dangerous situations if azide solutions should be directly disposed down the drain into a sanitary sewer system. Metal in the plumbing system could react, forming highly sensitive metal azide crystals which could accumulate over years. Adequate precautions are necessary for the safe and environmentally responsible disposal of azide solution residues.[27]
Intentional consumption
Sodium azide has gained attention in the Netherlands[28] and abroad[29] as a chemical used for homicidal and suicidal purposes.
Sodium azide has been attributed to at least 172 deaths in the period from 2015 to 2022 as part of an illicit substance used as a suicide aid commonly called drug X (Dutch: middel X)[30] In 2021, a review of all case reports of sodium azide intoxication indicated that 37% of cases were suicide attempts.[31] An increase in the usage of sodium azide as a suicide drug has been attributed to its availability through pyrotechnics-focused online stores.[32]
Treatment
The US CDC reports no specific antidote for azide poisoning.[33] A 2021 narrative review identifies several cases of survival from ingestion when the patient is treated with antidotes for cyanide poisoning. From a mechanistic standpoint, hydroxocobalamin is more likely to be helpful than other antidotes such as sodium nitrite and sodium thiosulfate. As a result, the recommended treatment is hemodynamic support and hydroxocobalamin. First responders should use personal protection equipment to protect themselves from azide exposure.[34]
References
- ↑ 1.0 1.1 1.2 Stevens E. D.; Hope H. (1977). "A Study of the Electron-Density Distribution in Sodium Azide, NaN3". Acta Crystallographica A 33 (5): 723–729. doi:10.1107/S0567739477001855.
- ↑ "Sodium azide". https://molekula.com/catalog/26628-22-8/31803515-Sodium%20azide.
- ↑ 3.0 3.1 3.2 NIOSH Pocket Guide to Chemical Hazards. "#0560". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0560.html.
- ↑ "Material Safety Data Sheet". Sciencelab.com. November 6, 2008. http://www.lamission.edu/lifesciences/MSDS/MSDS/SodiumAzide.pdf.
- ↑ 5.0 5.1 Jobelius, Horst H.; Scharff, Hans-Dieter (2000). Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a13_193. ISBN 9783527306732.
- ↑ Template:Wells1984
- ↑ Pringle, G. E.; Noakes, D. E. (1968-02-15). "The crystal structures of lithium, sodium and strontium azides". Acta Crystallographica Section B 24 (2): 262–269. doi:10.1107/s0567740868002062. Bibcode: 1968AcCrB..24..262P.
- ↑ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils, ed., Inorganic Chemistry, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5.
- ↑ Committee on Prudent Practices for Handling, Storage, and Disposal of Chemicals in Laboratories, Board on Chemical Sciences and Technology, Commission on Physical Sciences, Mathematics, and Applications, National Research Council (1995). "Disposal of Waste". Prudent Practices in the Laboratory: Handling and Disposal of Chemicals. Washington, DC: National Academy Press. p. 165. ISBN 978-0-309-05229-0. http://books.nap.edu/openbook.php?record_id=4911&page=165.
- ↑ 10.0 10.1 Turnbull, Kenneth; Narsaiah, B.; Yadav, J. S.; Yakaiah, T.; Lingaiah, B. P. V. (2008-03-14), "Sodium Azide", Encyclopedia of Reagents for Organic Synthesis, Chichester, UK: John Wiley & Sons, Ltd, doi:10.1002/047084289x.rs045.pub2, ISBN 978-0471936237
- ↑ Fedoroff, Basil T.; Aaronson, Henry A.; Reese, Earl F.; Sheffield, Oliver E.; Clift, George D. (1960). Encyclopedia Of Explosives And Related Items Vol. I. New Jersey, USA: U.S. Army Research And Development Command TACOM, ARDEC: Warheads, Energetics And Combat Support Center, Picatinny Arsenal. pp. A574-575. https://apps.dtic.mil/sti/tr/pdf/AD0257189.pdf?page=684.
- ↑ Betterton, E. A. (2003). "Environmental Fate of Sodium Azide Derived from Automobile Airbags". Critical Reviews in Environmental Science and Technology 33 (4): 423–458. doi:10.1080/10643380390245002. Bibcode: 2003CREST..33..423B.
- ↑ Halford, Bethany (November 15, 2022). "What chemicals make airbags inflate, and how have they changed over time?". Chemical & Engineering News 100 (41). https://cen.acs.org/safety/chemicals-make-airbags-inflate-changed/100/i41. Retrieved 4 June 2023. "The chemical reaction used to deploy airbags has evolved, but one iteration resulted in massive recalls".

- ↑ Rohloff John C. et al. (1998). "Practical Total Synthesis of the Anti-Influenza Drug GS-4104". J. Org. Chem. 63 (13): 4545–4550. doi:10.1021/jo980330q.
- ↑ Scopes, Robert K. (1994). Protein Purification. New York, NY: Springer New York. p. 204. doi:10.1007/978-1-4757-2333-5. ISBN 978-1-4419-2833-7.
- ↑ Deutscher, M.P. (1990). Guide to Protein Purification. Methods in enzymology. Academic Press. p. 729. ISBN 978-0-12-182083-1. https://books.google.com/books?id=zTiRJHpKIQoC&pg=PR11. Retrieved 2023-04-10.
- ↑ Lichstein, H. C.; Soule, M. H. (1943). "Studies of the Effect of Sodium Azide on Microbic Growth and Respiration: I. The Action of Sodium Azide on Microbic Growth". Journal of Bacteriology 47 (3): 221–230. doi:10.1128/JB.47.3.221-230.1944. PMID 16560767.
- ↑ Applications of sodium azide for control of soilborne pathogens in potatoes. Rodriguez-Kabana, R., Backman, P. A. and King, P.S., Plant Disease Reporter, 1975, Vol. 59, No. 6, pp. 528-532 (link)
- ↑ Awan, M. Afsar; Konzak, C. F.; Rutger, J. N.; Nilan, R. A. (2000-01-01). "Mutagenic Effects of Sodium Azide in Rice1". Crop Science 20 (5): 663–668. doi:10.2135/cropsci1980.0011183x002000050030x.
- ↑ Cheng, Xiongying; Gao, Mingwei (1988). "Biological and genetic effects of combined treatments of sodium azide, gamma rays and EMS in barley". Environmental and Experimental Botany 28 (4): 281–288. doi:10.1016/0098-8472(88)90051-2. Bibcode: 1988EnvEB..28..281C.
- ↑ Rines, H. W. (1985-02-01). "Sodium azide mutagenesis in diploid and hexaploid oats and comparison with ethyl methanesulfonate treatments". Environmental and Experimental Botany 25 (1): 7–16. doi:10.1016/0098-8472(85)90043-7. Bibcode: 1985EnvEB..25....7R.
- ↑ Chang, Soju; Lamm, Steven H. (2003-05-01). "Human Health Effects of Sodium Azide Exposure: A Literature Review and Analysis". International Journal of Toxicology 22 (3): 175–186. doi:10.1080/10915810305109. ISSN 1091-5818. PMID 12851150.
- ↑ "MSDS: sodium azide". Mallinckrodt Baker. 2008-11-21. http://hazard.com/msds/mf/baker/baker/files/s2906.htm.
- ↑ Olson, Kent; Anderson, Ilene B. (18 September 2006). Poisoning & Drug Overdose, 5th Edition. McGraw-Hill Companies, Incorporated. pp. 123. ISBN 978-0-07-144333-3. https://books.google.com/books?id=25avFCpfQAcC&pg=PA123.
- ↑ Gordon, Steven M.; Drachman, Jonathan; Bland, Lee A.; Reid, Marie H.; Favero, Martin; Jarvis, William R. (1990-01-01). "Kidney International - Abstract of article: Epidemic hypotension in a dialysis center caused by sodium azide". Kidney Int 37 (1): 110–115. doi:10.1038/ki.1990.15. ISSN 0085-2538. PMID 2299796.
- ↑ Kimura, Hiroshi; Mittal, Chandra K.; Murad, Ferid (1975-10-23). "Increases in cyclic GMP levels in brain and liver with sodium azide an activator of guanylate cyclase". Nature 257 (5528): 700–702. doi:10.1038/257700a0. PMID 241939. Bibcode: 1975Natur.257..700K.
- ↑ "Sodium Azide | Environmental Health & Safety | Northeastern University". https://www.northeastern.edu/ehs/ehs-programs/hazardous-waste-management/fact-sheets/sodium-azide/.
- ↑ Bruin, Maaike A. C.; Dekker, Douwe; Venekamp, Nikkie; Tibben, Matthijs; Rosing, Hilde; de Lange, Dylan W.; Beijnen, Jos H.; Huitema, Alwin D. R. (March 2021). "Toxicological analysis of azide and cyanide for azide intoxications using gas chromatography" (in en). Basic & Clinical Pharmacology & Toxicology 128 (3): 534–541. doi:10.1111/bcpt.13523. ISSN 1742-7835. PMID 33090684.
- ↑ Конец скорпиона // Аргументы и факты
- ↑ "Zeker 172 mensen overleden door zelfdoding met middel X" (in nl). 18 April 2024. https://www.nu.nl/gezondheid/6309490/zeker-172-mensen-overleden-door-zelfdoding-met-middel-x.html.
- ↑ Wachełko, Olga; Zawadzki, Marcin; Szpot, Paweł (2021-07-30). "A novel procedure for stabilization of azide in biological samples and method for its determination (HS-GC-FID/FID)" (in en). Scientific Reports 11 (1): 15568. doi:10.1038/s41598-021-95104-5. ISSN 2045-2322. PMID 34330976.
- ↑ van der Heijden, Lisa T.; van den Hondel, Karen E.; Olyslager, Erik J. H.; de Jong, Lutea A. A.; Reijnders, Udo J. L.; Franssen, Eric J. F. (2023-07-13). "Internet-Purchased Sodium Azide Used in a Fatal Suicide Attempt: A Case Report and Review of the Literature" (in en). Toxics 11 (7): 608. doi:10.3390/toxics11070608. ISSN 2305-6304. PMID 37505573. Bibcode: 2023Toxic..11..608V.
- ↑ "Sodium Azide" (in en-us). 6 September 2024. https://www.cdc.gov/chemical-emergencies/chemical-fact-sheets/sodium-azide.html.
- ↑ Tat, John; Heskett, Karen; Satomi, Shiho; Pilz, Renate B.; Golomb, Beatrice A.; Boss, Gerry R. (3 August 2021). "Sodium azide poisoning: a narrative review". Clinical Toxicology 59 (8): 683–697. doi:10.1080/15563650.2021.1906888. PMID 34128439.
External links
- International Chemical Safety Card 0950.
- NIOSH Pocket Guide to Chemical Hazards.
- R., Frances (2006). "Is there poison in auto air bags?". https://www.straightdope.com/columns/read/2681/is-there-poison-in-auto-air-bags/.
Salts and covalent derivatives of the azide ion
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HN3 | He | ||||||||||||||||||
| LiN3 | Be(N3)2 | B(N3)3 | CH3N3, C(N3)4 |
N(N3)3,H2N—N3 | O | FN3 | Ne | ||||||||||||
| NaN3 | Mg(N3)2 | Al(N3)3 | Si(N3)4 | P | SO2(N3)2 | ClN3 | Ar | ||||||||||||
| KN3 | Ca(N3)2 | Sc(N3)3 | Ti(N3)4 | VO(N3)3 | Cr(N3)3, CrO2(N3)2 |
Mn(N3)2 | Fe(N3)3 | Co(N3)2, Co(N3)3 |
Ni(N3)2 | CuN3, Cu(N3)2 |
Zn(N3)2 | Ga(N3)3 | Ge | As | Se(N3)4 | BrN3 | Kr | ||
| RbN3 | Sr(N3)2 | Y | Zr(N3)4 | Nb | Mo | Tc | Ru(N3)63− | Rh(N3)63− | Pd(N3)2 | AgN3 | Cd(N3)2 | In | Sn | Sb | Te | IN3 | Xe(N3)2 | ||
| CsN3 | Ba(N3)2 | Hf | Ta | W | Re | Os | Ir(N3)63− | Pt(N3)62− | Au(N3)4− | Hg2(N3)2, Hg(N3)2 |
TlN3 | Pb(N3)2 | Bi(N3)3 |
Po | At | Rn | |||
| Fr | Ra(N3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce(N3)3, Ce(N3)4 |
Pr | Nd | Pm | Sm | Eu | Gd(N3)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac | Th | Pa | UO2(N3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
 |

