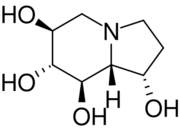
Chemistry:Castanospermine
| |
| Names | |
|---|---|
| Preferred IUPAC name
(1S,6S,7R,8R,8aR)-Octahydroindolizine-1,6,7,8-tetrol | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H15NO4 | |
| Molar mass | 189.209 g/mol |
| Appearance | White to off-white solid |
| Melting point | 212 to 215 °C (414 to 419 °F; 485 to 488 K) |
| Soluble | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H312, H332 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Castanospermine is an indolizidine alkaloid first isolated from the seeds of Castanospermum australe.[3] It is a potent inhibitor of some glucosidase enzymes[4] and has antiviral activity in vitro and in mouse models.[5]
The castanospermine derivative celgosivir is an antiviral drug candidate currently in development for possible use in treating hepatitis C virus (HCV) infection.[6]
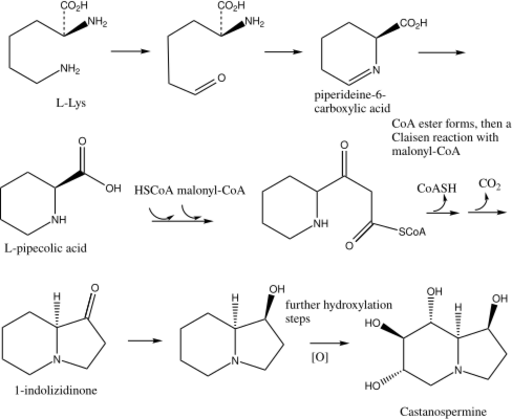
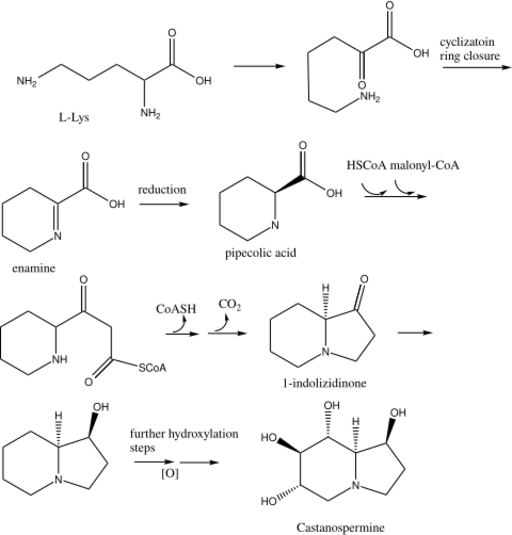
Biosynthesis of castanospermine
L-Lys undergoes a transamination to form α-aminoadipic acid. α-aminoadipic acid undergoes a ring closure and then a reduction to form L-pipecolic acid (Figure 1).[7] In the alternate pathway (Figure 2), L-Lys cyclizes and forms the enamine, which reduces to L-pipecolic acid.
HSCoA and then malonyl-CoA react in a Claisen reaction with L-pipecolic acid to form SCoA ester which undergoes a ring closure to form 1-indolizidinone. The carbonyl on 1-indolizidinone is reduced to the hydroxyl group. The molecule is then further hydroxylated to form the final product castanospermine.[8]
Biosynthesis shown in figure:[9][10]
See also
References
- ↑ Merck Index, 11th Edition, 1902.
- ↑ Castanospermine at Fermentek
- ↑ Hohenschutz, Liza D.; Bell, E. Arthur; Jewess, Phillip J.; Leworthy, David P.; Pryce, Robert J.; Arnold, Edward; Clardy, Jon (1981). "Castanospermine, a 1,6,7,8-tetrahydroxyoctahydroindolizine alkaloid, from seeds of Castanospermum australe". Phytochemistry 20 (4): 811–14. doi:10.1016/0031-9422(81)85181-3. Bibcode: 1981PChem..20..811H.
- ↑ R Saul; J J Ghidoni; R J Molyneux; A D Elbein (1985). "Castanospermine inhibits alpha-glucosidase activities and alters glycogen distribution in animals". PNAS 82 (1): 93–97. doi:10.1073/pnas.82.1.93. PMID 3881759. Bibcode: 1985PNAS...82...93S.
- ↑ "Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo". J Virol 79 (14): 8698–706. 2005. doi:10.1128/JVI.79.14.8698-8706.2005. PMID 15994763.
- ↑ Durantel, D. (2009). "Celgosivir, an alpha-glucosidase I inhibitor for the potential treatment of HCV infection". Current Opinion in Investigational Drugs 10 (8): 860–70. PMID 19649930.
- ↑ Hartmann, Michael; Kim, Denis; Bernsdorff, Friederike; Ajami-Rashidi, Ziba; Scholten, Nicola; Schreiber, Stefan; Zeier, Tatyana; Schuck, Stefan et al. (2017-03-22). "Biochemical Principles and Functional Aspects of Pipecolic Acid Biosynthesis in Plant Immunity". Plant Physiology 174 (1): 124–153. doi:10.1104/pp.17.00222. ISSN 0032-0889. PMID 28330936.
- ↑ Dewick, Paul (2009). Medicinal Natural Products A Biosynthetic Approach. United Kingdom: Wiley. pp. 330. ISBN 978-0-470-74167-2.
- ↑ Hartman, Michael (Summer 2018). "Biochemical Principles and Functional Aspects of Pipecolic Acid Biosynthesis in Plant Immunity". Plant Physiology 174 (1): 124–153. doi:10.1104/pp.17.00222. PMID 28330936.
- ↑ Walsh, Christopher (2017). Natural Product Biosynthesis: Chemical Logic and Enzymatic Machinery. Royal Society of Chemistry. pp. 270. ISBN 978-1788010764.
Dewick, Paul (2009). Medicinal Natural Product A Biosynthetic Approach. Wiley. ISBN 978-0-470-74168-9.
Michael, Denis; Hartmann, Kim; Bernsdorff, Friederike; Ajami-Rashidi, Ziba; Scholten, Nicola (May 2017). "Biochemical Principles and Functional Aspects of Pipecolic Acid Biosynthesis in Plant Immunity". Plant Physiology 174 (1): 124–153. doi:10.1104/pp.17.00222. PMID 28330936.
 |