Chemistry:Swainsonine
| |
| Names | |
|---|---|
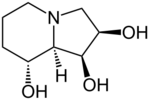
| Preferred IUPAC name
(1S,2R,8R,8aR)-Octahydroindolizine-1,2,8-triol | |
| Other names
Tridolgosir
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H15NO3 | |
| Molar mass | 173.2 |
| Melting point | 143 to 144 °C (289 to 291 °F; 416 to 417 K) |
| 10 mg/1 mL | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Swainsonine is an indolizidine alkaloid. It is a potent inhibitor of Golgi alpha-mannosidase II, an immunomodulator, and a potential chemotherapy drug.[1] As a toxin in locoweed (likely its primary toxin[2]) it also is a significant cause of economic losses in livestock industries, particularly in North America. It was first isolated from Swainsona canescens.[3]
Pharmacology
Swainsonine inhibits glycoside hydrolases, specifically those involved in N-linked glycosylation. Disruption of Golgi alpha-mannosidase II with swainsonine induces hybrid-type glycans. These glycans have a Man5GlcNAc2 core with processing on the 3-arm that resembles so-called complex-type glycans.[citation needed]
The pharmacological properties of this product have not been fully investigated.[citation needed]
Sources
| Family | Fungi |
|---|---|
| Pleosporaceae | Undifilum oxytropis[4] |
| Clavicipitaceae | Metarhizium anisopliae[5] |
| Family | Plants |
|---|---|
| Fabaceae | Swainsona canescens, Astragalus earlei, A. mollissimus, A. pubentissimus, A. lentiginosis, A. wootoni, A. nothoxys, A. tephrodes, A. humistratus[6][7][3] |
| Convolvulaceae | Jacquemontia corymbulosa, Ipomoea verbascoidea, I. subincana, I. megapotamica, I. rosea, I. carnea, I. sericophylla, I. riedelii[8][9][10][11] |

Biosynthesis
The biosynthesis of swainsonine has been investigated in the fungus Rhizoctonia leguminicola, and it initially involves the conversion of lysine into pipecolic acid. The pyrrolidine ring is then formed via retention of the carbon atom of the pipecolate's carboxyl group, as well as the coupling of two more carbon atoms from either acetate or malonate to form a pipecolylacetate. The retention of the carboxyl carbon is striking, since it is normally lost in the biosynthesis of most other alkaloids.[12]
The resulting oxoindolizidine is then reduced to (1R,8aS)- 1-hydroxyindolizidine, which is subsequently hydroxylated at the C2 carbon atom to yield 1,2-dihydroxyindolizidine. Finally, an 8-hydroxyl group is introduced through epimerization at C-8a to yield swainsonine. Schneider et al. have suggested that oxidation occurs at C-8a to give an iminium ion. Reduction from the β face would then yield the R configuration of swainsonine, as opposed to the S configuration of slaframine, another indolizidine alkaloid whose biosynthesis is similar to that of swainsonine during the first half of the pathway and also shown above alongside that of swainsonine. The instance at which oxidation and reduction occur with regard to the introduction of the hydroxyl groups at the C2 and C8 positions is still under investigation.[12]
The biosynthetic pathway of swainsonine has also been investigated in the Diablo locoweed (Astragalus oxyphysus). Through detection of (1,8a-trans)-1-hydroxyindolizidine and (1,8a-trans-1,2-cis)-1,2-dihydroxyindolizidine—two precursors of swainsonine in the fungus pathway—in the shoots of the plant, Harris et al. proposed that the biosynthetic pathway of swainsonine in the locoweed is nearly identical to that of the fungus.[12]
Synthesis
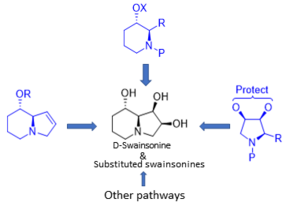
Despite the small size of swaisonine, the synthesis of this molecule and its analogues is quite challenging due to the presence of four chiral centers. In most cases, synthesis implies the use of sugars, chiral aminoacids as starting compounds, or chiral catalysts to induce chirality.The swainsonine synthesis was systemazed by three common precursors: 8-oxy-hexahydroindolizines, N-protected-3-oxy-2-substituted-piperidines and 2-substituted-pyrrolidine-3,4-protected-diols.[13]
Livestock losses
Because chronic intoxication with swainsonine causes a variety of neurological disorders in livestock,[14] these plant species are known collectively as locoweeds. Other effects of intoxication include reduced appetite and consequent reduced growth in young animals and loss of weight in adults, and cessation of reproduction (loss of libido, loss of fertility, and abortion).[15]
Potential uses
Swainsonine has a potential for treating cancers such as glioma[16] and gastric carcinoma.[17] However, a phase II clinical trial of GD0039 (a hydrochloride salt of swainsonine) in 17 patients with renal carcinoma was discouraging.[18] Swainsonine's activity against tumors is attributed to its stimulation of macrophages.[19]
Swainsonine also has potential uses as an adjuvant for anti-cancer drugs and other therapies in use. In mice, swainsonine reduces the toxicity of doxorubicin, suggesting that swainsonine might enable use of higher doses of doxorubicin.[20][21] Swainsonine may promote restoration of bone marrow damaged by some types of cancer treatments.[22][23]
Molecular mechanism
The inhibitory effect of swainsonine on Golgi Mannosidase II (GMII) was proposed to be due to its ability to bind in the GMII binding pocket in a similar fashion as the natural GMII substrate in its transition state.[24] Later, it was shown that the binding pattern of the swainsonine molecule resembles that of the Michaelis complex of mannose and only the protonated, charge positive swainsonine molecule binds similarly to the substrate in its transition state.[25] The actual state in which swainsonine binds in the mannosidase remains undetermined and is most likely dependent on the pH at which the enzyme operates.[25]
See also
- Castanospermine
- Mannosidosis
References
- ↑ "NCATS Inxight: Drugs" (in en). https://drugs.ncats.io/substances?q=%22Swainsonine%22.
- ↑ "The lesions of locoweed (Astragalus mollissimus), swainsonine, and castanospermine in rats". Veterinary Pathology 32 (3): 289–98. May 1995. doi:10.1177/030098589503200311. PMID 7604496.
- ↑ 3.0 3.1 "Inhibition of lysosomal alpha-mannosidase by swainsonine, an indolizidine alkaloid isolated from Swainsona canescens". The Biochemical Journal 191 (2): 649–51. November 1980. doi:10.1042/bj1910649. PMID 6786280.
- ↑ Lu, Hao; Quan, Haiyun; Ren, Zhenhui; Wang, Shuai; Xue, Ruixu; Zhao, Baoyu (2016). "The Genome of Undifilum oxytropis Provides Insights into Swainsonine Biosynthesis and Locoism". Scientific Reports 6 (1): 30760. doi:10.1038/srep30760. ISSN 2045-2322. PMID 27477109. Bibcode: 2016NatSR...630760L.
- ↑ Sim, Kim Lan; Perry, David (1 September 1995). "Swainsonine production by Metarhizium anisopliae determined by means of an enzymatic assay". Mycological Research 99 (9): 1078–1082. doi:10.1016/S0953-7562(09)80776-4.
- ↑ "Swainsonine and endophyte relationships in Astragalus mollissimus and Astragalus lentiginosus". Journal of Agricultural and Food Chemistry 59 (4): 1281–7. February 2011. doi:10.1021/jf103551t. PMID 21214242.
- ↑ Gardner, DR; Cook, D (2016). "Analysis of Swainsonine and Swainsonine N-Oxide as Trimethylsilyl Derivatives by Liquid Chromatography-Mass Spectrometry and Their Relative Occurrence in Plants Toxic to Livestock". J Agric Food Chem 64 (31): 6156–62. doi:10.1021/acs.jafc.6b02390. PMID 27436221.
- ↑ "Production of the alkaloid swainsonine by a fungal endosymbiont of the Ascomycete order Chaetothyriales in the host Ipomoea carnea". Journal of Agricultural and Food Chemistry 61 (16): 3797–803. April 2013. doi:10.1021/jf4008423. PMID 23547913.
- ↑ Barbosa, Rossemberg C.; Riet-Correa, Franklin; Lima, Everton F.; Medeiros, Rosane M.T.; Guedes, Karla M.R.; Gardner, Dale R.; Molyneux, Russell J.; Melo, Lúcio E.H. de (2007). "Experimental swainsonine poisoning in goats ingesting Ipomoea sericophylla and Ipomoea riedelii (Convolvulaceae)". Pesquisa Veterinária Brasileira 27 (10): 409–414. doi:10.1590/S0100-736X2007001000004. ISSN 0100-736X.
- ↑ Mendonça, Fábio S.; Silva Filho, Givaldo B.; Chaves, Hisadora A.S.; Aires, Lorena D.A.; Braga, Thaiza C.; Gardner, Dale R.; Cook, Daniel; Buril, Maria T. (2018). "Detection of swainsonine and calystegines in Convolvulaceae species from the semiarid region of Pernambuco". Pesquisa Veterinária Brasileira 38 (11): 2044–2051. doi:10.1590/1678-5150-pvb-5945. ISSN 1678-5150.
- ↑ "Alpha-mannosidosis in goats caused by the swainsonine-containing plant Ipomoea verbascoidea". Journal of Veterinary Diagnostic Investigation 24 (1): 90–5. January 2012. doi:10.1177/1040638711425948. PMID 22362938.
- ↑ 12.0 12.1 12.2 Harris, Constance M.; Bruce C. Campbell; Russell J. Molyneux; Thomas M. Harris (1988). "Biosynthesis of swainsonine in the diablo locoweed (Astragalus oxyphyrus)". Tetrahedron Letters 29 (38): 4815–4818. doi:10.1016/S0040-4039(00)80616-4.

- ↑ 13.0 13.1 Drogalin, Artem (2022-07-21). "Advances in the Chemistry of (−)-D-Swainsonine" (in en). ChemistrySelect 7 (27). doi:10.1002/slct.202201905. ISSN 2365-6549. https://onlinelibrary.wiley.com/doi/10.1002/slct.202201905.
- ↑ "THE DARLING PEA.". The Sydney Morning Herald (National Library of Australia): p. 5. 14 May 1897. http://nla.gov.au/nla.news-article14103431.
- ↑ "Locoweeds: effects on reproduction in livestock". Journal of Natural Toxins 8 (1): 53–62. February 1999. PMID 10091128.
- ↑ "Suppressive effects of swainsonine on C6 glioma cell in vitro and in vivo". Phytomedicine 16 (11): 1070–4. May 2009. doi:10.1016/j.phymed.2009.02.012. PMID 19427771.
- ↑ "Inhibition of the growth of human gastric carcinoma in vivo and in vitro by swainsonine". Phytomedicine 14 (5): 353–9. May 2007. doi:10.1016/j.phymed.2006.08.003. PMID 17097281.
- ↑ "Phase II study of the efficacy and safety of oral GD0039 in patients with locally advanced or metastatic renal cell carcinoma". Investigational New Drugs 23 (6): 577–81. December 2005. doi:10.1007/s10637-005-0793-z. PMID 16034517.
- ↑ "Activation of resident tissue-specific macrophages by swainsonine". Oncology Research 7 (9): 425–33. 1995. PMID 8835286.
- ↑ "Mice primed with swainsonine are protected against doxorubicin-induced lethality". Cellular and Molecular Biology (Noisy-le-Grand, France) 49 (7): 1089–99. November 2003. PMID 14682391.
- ↑ "Coadministration of swainsonine and doxorubicin attenuates doxorubicin-induced lethality in mice". Cellular and Molecular Biology (Noisy-le-Grand, France) 49 (7): 1037–48. November 2003. PMID 14682385.
- ↑ "Limits of stimulation of proliferation and differentiation of bone marrow cells of mice treated with swainsonine". International Immunopharmacology 3 (10–11): 1537–47. October 2003. doi:10.1016/S1567-5769(03)00186-3. PMID 12946451.
- ↑ "Swainsonine protects both murine and human haematopoietic systems from chemotherapeutic toxicity". British Journal of Cancer 80 (1–2): 87–95. April 1999. doi:10.1038/sj.bjc.6690326. PMID 10389983.
- ↑ Petersen, Luis; Ardèvol, Albert; Rovira, Carme; Reilly, Peter J. (23 June 2010). "Molecular Mechanism of the Glycosylation Step Catalyzed by Golgi α-Mannosidase II: A QM/MM Metadynamics Investigation". Journal of the American Chemical Society 132 (24): 8291–8300. doi:10.1021/ja909249u. PMID 20504027. https://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=1009&context=cbe_pubs.
- ↑ 25.0 25.1 Sladek, V.; Kóňa, J.; Tokiwa, H. (2017). "In silico analysis of interaction pattern switching in ligand⋯receptor binding in Golgi α-mannosidase II induced by the protonated states of inhibitors". Physical Chemistry Chemical Physics 19 (19): 12527–12537. doi:10.1039/c7cp01200d. PMID 28470253. Bibcode: 2017PCCP...1912527S.
 |
