Chemistry:Solanidine
| |
| Names | |
|---|---|
| IUPAC name
Solanid-5-en-3β-ol
| |
| Systematic IUPAC name
(2S,4aR,4bS,6aS,6bR,7S,7aR,10S,12aS,13aS,13bS)-4a,6a,7,10-Tetramethyl-2,3,4,4a,4b,5,6,6a,6b,7,7a,8,9,10,11,12a,13,13a,13b,14-icosahydro-1H-naphtho[2′,1′:4,5]indeno[1,2-b]indolizin-2-ol | |
| Other names
Solatubin; Solatubine
| |
| Identifiers | |
3D model (JSmol)
|
|
| 45370 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H43NO | |
| Molar mass | 397.647 g·mol−1 |
| Hazards[1] | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H413 | |
| P264, P270, P273, P301+312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
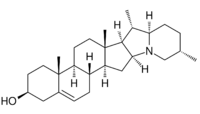
Solanidine is a poisonous steroidal alkaloid chemical compound that occurs in plants of the family Solanaceae, such as potato and Solanum americanum.[2][3] Human ingestion of solanidine also occurs via the consumption of the glycoalkaloids, α-solanine and α-chaconine, present in potatoes.[4][5] The sugar portion of these glycoalkaloids hydrolyses in the body, leaving the solanidine portion.[5] Solanidine occurs in the blood serum of normal healthy people who eat potato, and serum solanidine levels fall markedly once potato consumption ceases.[6] Solanidine from food is also stored in the human body for prolonged periods of time, and it has been suggested that it could be released during times of metabolic stress with the potential for deleterious consequences.[7] Solanidine is responsible for neuromuscular syndromes via cholinesterase inhibition.[8][9]
Uses
Solanidine is a very important precursor for the synthesis of hormones and some pharmacologically active compounds.[2]
Synthetic uses
Solanidine to 16-DPA conversion
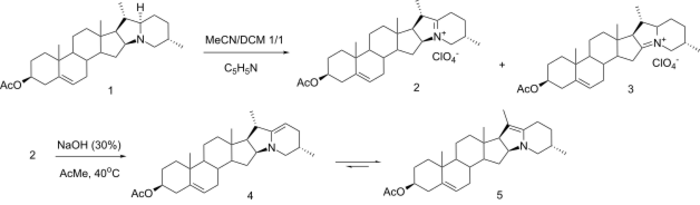
In 1994, Gunic and coworkers reported the electrochemical oxidation of 3β-acetoxy-solanidine in CH3CN/CH2Cl2 1/1 with pyridine as a base. The corresponding iminium salts 2 and 3 were obtained in a 1/1 ratio in good yield. Performing this electrochemical reaction in DCM with pyridine gives 3 in 95% yield, while the same reaction in acetone gives iminium salt 2 in 95% yield. Iminium ion 2 can be isomerized to the thermodynamically more stable enamine 5. THis isomerization is believed to proceed via enamine 4, which is the kinetic product.
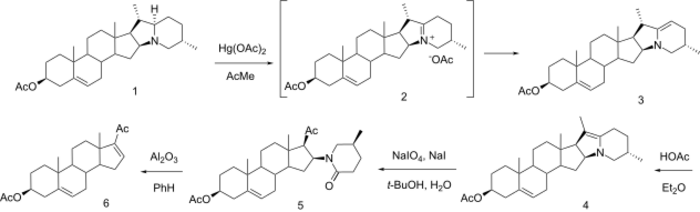
In 1997, Gaši et al. reported a short procedure for the degradation of solanidine to 16-Dehydropregnenolone acetate. Instead of applying the electrochemical oxidation, Hg(OAc)2 in acetone was used as oxidizing agent. The advantage of this reagent and solvent system was the ease of use and the selective formation of iminium salt 2, which spontaneously isomerized to enamine 3 (94%). This enamine was then subjected to another isomerization, which yielded the more thermodynamically more stable enamine 4. NaIO4-oxidation opened up the cyclic enamine and gave lactam 5. Elimination of the lactam part with Al2O3 in benzene afforded in 34% 16-dehydropregnenolone acetate (DPA) (6). Using K2CO3 in benzene followed by reacetylation produced 6 in a lower yield (11%).
Solanidine to tomatidenol conversion
In 1968, Beisler and Sato synthesized tomatidenol from solanidine, and reported the successful opening of the E ring of solanidine via the von Braun reaction.[12][13] Only in case of acetylated solanidine the von Braun reaction gave the E ring-opened product in 78% yield.

Treatment of α-bromine with KOAc gave in good yield the β-diacetate, which could be reduced with red-Al in benzene.
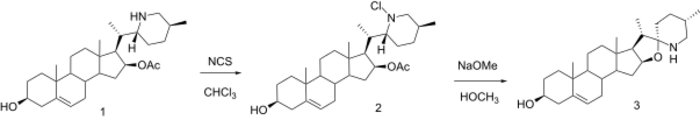
These types of compounds can be ringclosed to spirosolane compounds as shown by Schreiber.
See also
References
- ↑ "Solanidine" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/65727#section=Safety-and-Hazards.
- ↑ 2.0 2.1 Nikolic, NC; Stankovic, MZ (2003). "Solanidine hydrolytic extraction and separation from the potato (Solanum tuberosum L.) vines by using solid-liquid-liquid systems". Journal of Agricultural and Food Chemistry 51 (7): 1845–9. doi:10.1021/jf020426s. PMID 12643640.
- ↑ Mohy-ud-dint, A., Khan,Z., Ahmad, M., Kashmiri, M.A. (2010). "Chemotaxonomic value of alkaloids in Solanum nigrum complex". Pakistan Journal of Botany 42 (1): 653–660. http://www.pakbs.org/pjbot/PDFs/42(1)/PJB42(1)653.pdf.
- ↑ Friedman, M; Henika, PR; MacKey, BE (2003). "Effect of feeding solanidine, solasodine and tomatidine to non-pregnant and pregnant mice". Food and Chemical Toxicology 41 (1): 61–71. doi:10.1016/s0278-6915(02)00205-3. PMID 12453729. https://zenodo.org/record/1259981.
- ↑ 5.0 5.1 Kuiper-Goodman, T., Nawrot, P.S., Solanine and Chaconine, IPCS Inchem
- ↑ Harvey, M.H.; McMillan, M.; Morgan, M.R.A.; Chan, H. W. S. (1985). "Solanidine is Present in Sera of Healthy Individuals and in Amounts Dependent on their Dietary Potato Consumption". Human & Experimental Toxicology 4 (2): 187–194. doi:10.1177/096032718500400209. PMID 4007882.
- ↑ Claringbold, W. D. B.; Few, J. D.; Renwick, J. H. (1982). "Kinetics and retention of solanidine in man". Xenobiotica 12 (5): 293–302. doi:10.3109/00498258209052469. PMID 7135998.
- ↑ Bushway, R.J., Savage, S.A., Ferguson, B.S., Inhibition of acetyl cholinesterase by solanaceous glycoalkaloids and alkaloids, American Potato Journal, Aug. 1987, Volume 64, Issue 8, pp 409-413 [1]
- ↑ Everist, S.L., Poisonous Plants of Australia, Angus and Robertson, 1974, ISBN:0207142289.
- ↑ Gunic, E.; Tabakovic, I.; Gasi, K. M.; Miljkovic, D.; Juranic, I. (1994). "Products and Mechanisms in the Anodic Oxidation of Solanidine-Type Steroidal Alkaloids". The Journal of Organic Chemistry 59 (6): 1264–1269. doi:10.1021/jo00085a011.
- ↑ "16-Dehydropregnenolone acetate from solanidine". Journal of the Serbian Chemical Society 62 (6). 1996-11-04. https://www.shd.org.rs/HtDocs/SHD/Vol62/V62no6ad.htm. Retrieved 2023-03-14.
- ↑ Beisler, J. A.; Sato, Y. (1968). "A degradation of the solonidane skeleton". Chemical Communications (16): 963–964. doi:10.1039/C19680000963.
- ↑ Beisler, J. A.; Sato, Y. (1971). "Chemistry of the solanidane ring system". Journal of the Chemical Society C: Organic: 149–152. doi:10.1039/J39710000149.
- ↑ Schramm, Geza & Horst Riedl, "Verfahren zur Herstellung von Piperidylsteroiden [Process for the manufacture of piperidyl steroids]", DE patent 20217610, published 1971-11-25
- ↑ Schreiber, Klaus; Rönsch, Hasso (1965). "Solanum-Alkaloide, XLIV über Tomatid-5-en-3ß-ol aus Solanum dulcamara L. Und dessen Abbau zu 3ß-Acetoxy-pregna-5.16-dien-20-on". Justus Liebigs Annalen der Chemie 681: 187–195. doi:10.1002/jlac.19656810127.
 |