Chemistry:1-Nitroso-2-naphthol
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H7NO2 | |
| Molar mass | 173.171 g·mol−1 |
| Appearance | Yellowish-brown |
| Melting point | 109.5 °C (229.1 °F; 382.6 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H302, H315, H319, H335, H400 | |
| P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P270, P271, P273, P280, P301+317Script error: No such module "Preview warning".Category:GHS errors, P302+352, P304+340, P305+351+338, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P330, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
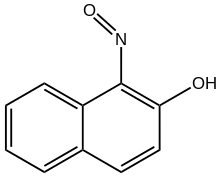
1-Nitroso-2-naphthol is an organic compound with the formula C
10H
6(NO)OH. It is one of several possible nitrosonaphthols, and the most studied for applications as an indicator and a dye.[2]
Synthesis and reactions
1-Nitroso-2-naphthol can be prepared by treatment of 2-naphthol with nitrous acid:[3]
- C
10H
7OH + HNO
2 → C
10H
6(NO)OH + H
2O
Its conjugate base forms deeply colored complexes with iron(II) and cobalt(II), complexes [M(C10H6(NO)O)3]2-.[4] The deep colors of these complexes results from the delocalized bonding within each five-membered chelate ring. These species can be classified as nitroso complexes.
See also
- Naphthol Green B, the iron complex of a sulfonated derivative of 1-nitroso-2-naphthol
References
- ↑ "1-Nitroso-2-naphthol" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/8580#section=Safety-and-Hazards.
- ↑ Gledhill, Martha; Van Den Berg, Constant M.G. (1994). "Determination of complexation of iron(III) with natural organic complexing ligands in seawater using cathodic stripping voltammetry". Marine Chemistry 47 (1): 41–54. doi:10.1016/0304-4203(94)90012-4. Bibcode: 1994MarCh..47...41G.
- ↑ Marvel, C. S.; Porter, P. K. (1922). "Nitroso-β-Naphthol". Organic Syntheses 2: 61. doi:10.15227/orgsyn.002.0061.
- ↑ Wang, Xiao; Zhang, Tianyong; Li, Bin; Yang, Qiusheng; Jiang, Shuang (2014). "Efficient hydroxylation of aromatic compounds catalyzed by an iron(II) complex with H2O2". Applied Organometallic Chemistry 28 (9): 666–672. doi:10.1002/aoc.3178.
 |

