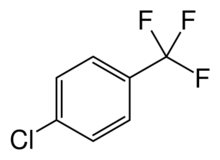
Chemistry:Parachlorobenzotrifluoride
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Chloro-4-(trifluoromethyl)benzene | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | PCBTF |
| 510203 | |
| ChemSpider | |
| EC Number |
|
| MeSH | C037723 |
PubChem CID
|
|
| UNII | |
| UN number | 2234 |
| |
| |
| Properties | |
| C7H4ClF3 | |
| Molar mass | 180.55 g·mol−1 |
| Appearance | Colorless liquid |
| Melting point | −36 °C (−33 °F; 237 K) |
| Boiling point | 139 °C (282 °F; 412 K) |
| 0 | |
| Vapor pressure | 7.9 |
Henry's law
constant (kH) |
0.0347 |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Warning |
| H226, H315, H319, H335, H411 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P391, P403+233, P403+235, P405 | |
| NFPA 704 (fire diamond) | |
| Flash point | 43 °C (109 °F; 316 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Parachlorobenzotrifluoride is a synthetic halogenated organic chemical compound with the molecular formula C7H4ClF3. It is frequently abbreviated PCBTF. Parachlorobenzotrifluoride is a colorless liquid with a distinct aromatic odor. PCBTF has been commercially-produced since the 1960s, initially as an intermediate in the production of other petrochemicals. But since the 1990s, it has been primarily used as a solvent.[1]
History
Occidental Chemical Corporation was a leading producer and sold it as part of its Oxsol® product line, specifically under the brand name of Oxsol 100.[2] Occidental Chemical Corporation sold the OXSOL line to an Israeli company, Makhteshim Agan Industries, Ltd., in 2002.[3]
Uses
PCBTF is increasingly used as a xylene replacement in cleaners, thinners, and other aromatic hydrocarbon blends.[1]
PCBTF is used as a component (5-12%) of low volatile organic compound (VOC) compliant polyurethane finishes.[4]
The substance is used as an ink solvent in the printing industry. Parachlorobenzotrifluoride has a high capacity for dissolving many inks used by the printing industry. In most cases, up to 22 grams of ink can be dissolved in 20 grams of PCBTF.[citation needed] An added benefit is that parachlorobenzotrifluoride dissolves most inks faster than toluene.
Health and Environmental effects
Health effects:
- Points of entry: eyes, ingestion, inhalation, skin.
- Target organs: central nervous system, kidneys, liver.
- Irritancy: eyes, respiratory tract, skin[2]
In the troposphere, PCBTF has an estimated half-life of 67 days. It is transformed by reaction with photochemically-produced hydroxyl radicals to give mainly 2-chloro-5-trifluoromethylphenol.[1]
Regulation
PCBTF currently has VOC Exempt status from the U.S. Environmental Protection Agency.[5] However, California's Office of Environmental Health Hazard Assessment (OEHHA) has adopted inhalation risk factors for PCBTF as of June 2019, which could have implications for its ongoing VOC Exempt status.[6][7]
References
- ↑ 1.0 1.1 1.2 Rayner-Canham, Geoff (March 2014). "Para-Chlorobenzotrifluoride (PCBTF) – the 'super-solvent'". Chem 13 News Magazine (The University of Waterloo). https://uwaterloo.ca/chem13-news-magazine/march-2014/chemistry/para-chlorobenzotrifluoride-pcbtf-super-solvent.
- ↑ 2.0 2.1 MSDS provided by Islechem
- ↑ "Trifluoromethylbenzene 98-08-8". National Cancer Institute. https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/trifluoromethylbenzene1_508.pdf.
- ↑ see MSDS for MINWAX product numbers 13025(5%) and 71029(12%) [full citation needed]
- ↑ "EPA Exempt Volatile Organic Compound: Parachlorobenzotrifluoride" (in en-US). 2018-01-30. https://www.govinfo.gov/content/pkg/FR-1994-10-05/pdf/FR-1994-10-05.pdf#page=25.
- ↑ "Chemical Listed Effective June 28, 2019 as Known to the State Of California To Cause Cancer: P-Chloro-a,a,a-Trifluorotoluene (Para-Chlorobenzotrifluoride, PCBTF)". https://oehha.ca.gov/proposition-65/crnr/chemical-listed-effective-june-28-2019-known-state-california-cause-cancer.
- ↑ p-Chloro-α,α,α-trifluorotoluene (p-Chlorobenzotrifluoride, PCBTF) - Cancer Inhalation Unit Risk Factor Scientific Review Panel Draft - January 2020 - California Office of Environmental Health Hazard Assessment
 |


