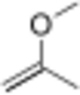
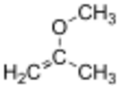
Chemistry:2-Methoxypropene
From HandWiki
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methoxyprop-1-ene | |||
| Other names
Methyl isopropenyl ether
Isopropenyl methyl ether | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C4H8O | |||
| Molar mass | 72.107 g·mol−1 | ||
| Appearance | Colorless liquid[1] | ||
| Density | 0.753 g/mL[2] | ||
| Boiling point | 34 to 36 °C (93 to 97 °F; 307 to 309 K)[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
2-Methoxypropene is an ether with the chemical formula C4H8O. It is a reagent used in organic synthesis to introduce a protecting group for alcohols,[1] and the conversion diols to the acetonide group.[3]
2-Methoxypropene can be prepared by the elimination of methanol from dimethoxypropane,[4] or by the addition of methanol to propyne or allene.[5]
References
- ↑ 1.0 1.1 Whitaker, K. Sinclair; Whitaker, D. Todd (2001). "2-Methoxypropene". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rm124. ISBN 0471936235.
- ↑ 2.0 2.1 2-Methoxypropene at Sigma-Aldrich
- ↑ Theodora W. Greene; Peter G. M. Wuts. Protective Groups in Organic Synthesis (3rd ed.). pp. 207–215.
- ↑ Newman, Melvin S.; Vander Zwan, Michael C. (1973). "Improved synthesis of 2-methoxypropene". The Journal of Organic Chemistry 38 (16): 2910. doi:10.1021/jo00956a040.
- ↑ Agré, B. A.; Taber, A. M.; Beregovykh, V. V.; Klebanova, F. D.; Nekrasov, N. V.; Sobolev, O. B.; Kalechits, I. V. (1983). "Kinetics of the catalytic synthesis of 2-methoxypropene". Pharmaceutical Chemistry Journal 17 (3): 221. doi:10.1007/BF00765172.
 |