Chemistry:Iodosilane
From HandWiki
| |||
|
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
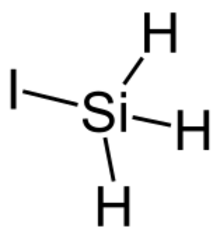
| SiH3I | |||
| Molar mass | 158.014 g/mol | ||
| Appearance | colorless crystals[1] | ||
| Melting point | −56.6 °C (216.6 K)[2] | ||
| Boiling point | 45.8 °C (318.9 K)[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Iodosilane is a chemical compound of silicon, hydrogen, and iodine. It is a colorless monoclinic crystal of space group P21/c at −157 °C.[1]
Preparation
Iodosilane is the first product of the reaction between monosilane and iodine, the other products being di-, tri- and finally tetraiodosilane (silicon tetraiodide).
It can also be produced by the reaction of phenylsilane or chlorophenylsilane with hydrogen iodide.[3]
- [math]\displaystyle{ \mathrm{ClC_6H_4SiH_3 + HI \longrightarrow C_6 H_5 Cl + SiH_3I} }[/math]
Properties
At low temperatures, iodosilant quickly reacts with [Co(CO)4]− to form SiH3Co(CO)4.[4]
Further reading
- Nakagawa, Jun; Hayashi, Michiro (1982). "Microwave spectra in the ν3, 2ν3, and ν6 excited states of iodosilane". Journal of Molecular Spectroscopy 93 (2): 441–444. doi:10.1016/0022-2852(82)90181-3. Bibcode: 1982JMoSp..93..441N.
- Ward, Laird G. L.; Norman, Arlan D.; Gondal, S. K.; MacDiarmid, A. G. (1968). "Bromosilane, iodosilane, and trisilylamine". in Jolly, William L.. Inorganic Syntheses. 11. McGraw-Hill. pp. 159–170. doi:10.1002/9780470132425.ch35. ISBN 978-0-470-13170-1.
- Sharbaugh, A. H.; Heath, G. A.; Thomas, L. F.; Sheridan, J. (1953). "Microwave spectrum and structure of iodosilane". Nature 171 (4341): 87. doi:10.1038/171087a0. Bibcode: 1953Natur.171...87S.
References
- ↑ 1.0 1.1 A. J. Blake, E. A. V. Ebsworth, S. G. D. Henderson, A. J. Welch (1988-08-15). "Structure of silyl iodide at 116 K". Acta Crystallographica Section C Crystal Structure Communications 44 (8): 1337–1339. doi:10.1107/S0108270188001155. http://scripts.iucr.org/cgi-bin/paper?S0108270188001155. Retrieved 2019-02-25.
- ↑ 2.0 2.1 A. G. Maddock, C. Reid, H. J. Emelus (August 1939). "New Iodine and Fluorine Derivatives of Monosilane" (in en). Nature 144 (3642): 328. doi:10.1038/144328a0. ISSN 0028-0836. Bibcode: 1939Natur.144Q.328M. http://www.nature.com/articles/144328a0. Retrieved 2019-02-25.
- ↑ (in de) Handbuch der präparativen anorganischen Chemie / 1.. Stuttgart. p. 686. ISBN 3-432-02328-6. OCLC 310719485.
- ↑ B. J. Aylett, J. M. Campbell (1965). "A volatile silicon–transition-metal compound" (in en). Chem. Commun. (London) (11): 217. doi:10.1039/C19650000217. ISSN 0009-241X.
 |