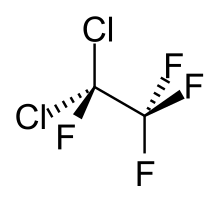
Chemistry:1,1-Dichlorotetrafluoroethane
| |

| |
| Names | |
|---|---|
| IUPAC name
1,1-dichloro-1,2,2,2-tetrafluoroethane
| |
| Other names
R114a; CFC-114a
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2Cl2F4 | |
| Molar mass | 170.92 g·mol−1 |
| Density | 1.455 g/cu cm (as a liquid under pressure) |
| Melting point | −56.6 °C (−69.9 °F; 216.6 K) |
| Boiling point | 3.4 °C (38.1 °F; 276.5 K) |
| 137 mg/L | |
| Solubility | benzene, diethyl ether, ethanol |
| log P | 2.78 |
| Vapor pressure | 1640 mm Hg |
Refractive index (nD)
|
1.3092 at 0 °C |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H335, H336, H370, H420 | |
| P260, P261, P264, P270, P271, P304+340, P308+316Script error: No such module "Preview warning".Category:GHS errors, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P403+233, P405, P501, P502 | |
| Related compounds | |
Related compounds
|
CFC-114 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1,1-Dichlorotetrafluoroethane is a chlorofluorocarbon also known as CFC-114a or R114a by American Society of Heating, Refrigerating, and Air Conditioning Engineers.[2] It has two chlorine atoms on one carbon atom and none on the other. It is one of two isomers of dichlorotetrafluoroethane, the other being 1,2-dichlorotetrafluoroethane, also known as CFC-114.
Formation
Given the ban on producing chlorofluorocarbons, it is actually important not to produce 1,1-dichlorotetrafluoroethane intentionally or unintentionally.
1,1-Dichlorotetrafluoroethane can be made free from other isomers by reacting trichlorotrifluoroethane (CFC-113 or CFC-113a) with antimony pentachloride.[3] Trichlorotrifluoroethane can also be reacted with sulfur tetrafluoride or dichlorodifluoromethane with aluminium fluoride catalyst to yield 1,1-dichlorotetrafluoroethane. The use of aluminium in the catalyst favours the asymmetric molecules.[4]
It can also be made in a reaction of tetrachloroethylene with hydrogen fluoride and chlorine, but this results in a mixture.[3]
Fluorinating 1,2-dichlorodifluoroethylene with fluorine produces a small amount of 1,1-dichlorotetrafluoroethane, but mostly tetrachlorotetrafluorobutene and some other chloroflurocarbons, so is not a good way.[5]
Properties
1,1-Dichlorotetrafluoroethane has a close boiling point (3.6°C) to the isomer 1,2-dichlorotetrafluoroethane (3.8°C), and so is difficult to separate by distillation.[6] Also in a gas chromatograph, it is hard to distinguish from the symmetric 1,2 isomer.[6]
Critical properties include critical temperature 145.7°C, critical pressure 4.92 Mpa and critical density of 0.82 g/ml.[7]
1,1-Dichlorotetrafluoroethane does not ignite in air.[7]
Reactions
1,1-Dichlorotetrafluoroethane reacts with hydrogen when heated at 300 to 600°C with a palladium catalyst in a hydrodechlorination. The main reaction product is 1,1,1,2-tetrafluoroethane, but also 1-chloro-1,2,2,2-tetrafluoroethane (CF3CHClF) and 1,1,1-trifluoroethane are formed.[8]
1,1-Dichlorotetrafluoroethane reacts with alkali metals, alkaline earths and aluminium.[7]
When heated with hydrogen over a nickel catalyst, 1,1-dichlorotetrafluoroethane is dechlorinated with replacement by hydrogen to yield a mixture of CF3CHClF and the dimer CF3CClFCClFCF3.[9]
Use
CFC-114a was used in aerosol propellants, blowing agents, and in polyolefin foams. There was also use in refrigerants. Production was banned in by the Montreal Protocol.[10]
CFC-114a is a possible intermediate in the production of HFC-134a[10] which can be produced by hydrogenation.[11]
Atmosphere
The ozone depletion potential of 1,1-dichlorotetrafluoroethane is 0.72.[12] The estimated lifetime in the atmosphere is about 100 years.[12] The radiative efficiency is 0.28 Wm−2ppb−1.[12] Global warming potential in 20 years is 6750.[12] The atmospheric concentration of CFC-114a is not usually measured separately from CFC-114 due to difficulties in distinguishing them apart.[12]
In 1978 atmospheric levels of CFC-114a were 0.35 ppt. By 2020 the level was up to 1.13 ppt.[13] CFC-114a appears to be emitted into the atmosphere is South East Asia.[10]
The atmospheric natural destruction of CFC-114a is by reaction with atomic oxygen, or breakup by ultraviolet light.[10] As of 2014 about 250 tons per year of CFC-114a were being put into the atmosphere.[10]
References
- ↑ "1,1-Dichloro-1,2,2,2-tetrafluoroethane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/1_1-Dichloro-1_2_2_2-tetrafluoroethane#section=Safety-and-Hazards.
- ↑ Deiters, Ulrich K (May 1997). "Some remarks on the nomenclature of refrigerants". Fluid Phase Equilibria 132 (1–2): 265–270. doi:10.1016/S0378-3812(96)03232-3.
- ↑ 3.0 3.1 Gumprecht, William Henry; Longoria, John Mark; Christoph, Frank J. (8 May 1991). "Process for manufacture of 1,1-dichlorotetrafluoroethane" (in en). https://patents.google.com/patent/EP0426343A1/en.
- ↑ Bozorgzadeh, H; Kemnitz, E; Nickkho-Amiry, M; Skapin, T; Winfield, J.M (January 2001). "Conversion of 1,1,2-trichlorotrifluoroethane to 1,1,1-trichlorotrifluoroethane and 1,1-dichlorotetrafluoroethane over aluminium-based catalysts". Journal of Fluorine Chemistry 107 (1): 45–52. doi:10.1016/S0022-1139(00)00350-X.
- ↑ Haszeldine, R. N. (1952). "849. Fluoro-olefins. Part I. The synthesis of hexafluorobuta-1 : 3-diene". Journal of the Chemical Society (Resumed): 4423. doi:10.1039/JR9520004423.
- ↑ 6.0 6.1 Chen, Limin; Makide, Yoshihiro; Tominaga, Takeshi (1994). "Determination of 1,2-dichlorotetrafluoroethane (CFC-114) Concentration in the Atmosphere". Chemistry Letters 23 (3): 571–574. doi:10.1246/cl.1994.571.
- ↑ 7.0 7.1 7.2 Bruno, Thomas J. (1990) (in en). Spectroscopic Library for Alternative Refrigerant Analysis. U.S. Department of Commerce, National Institute of Standards and Technology. pp. 25–27. https://books.google.com/books?id=PuI9AQAAIAAJ&dq=%221,1-Dichlorotetrafluoroethane%22&pg=PA1.
- ↑ Karpinski, Zbigniew; Early, Kintu; d'Itri, Julie L. (December 1996). "Catalytic Hydrodechlorination of 1,1-Dichlorotetrafluoroethane by Pd/Al2O3". Journal of Catalysis 164 (2): 378–386. doi:10.1006/jcat.1996.0394.
- ↑ Tomioka, Satoshi; Mori, Tohru; Ueda, Wataru; Morikawa, Yutaka; Ikawa, Tsuneo (October 1991). "A Novel Hydrodechlorinative Dimerization of Chlorofluorocarbons over Supported Ni Catalysts". Chemistry Letters 20 (10): 1825–1826. doi:10.1246/cl.1991.1825.
- ↑ 10.0 10.1 10.2 10.3 10.4 Laube, Johannes C.; Mohd Hanif, Norfazrin; Martinerie, Patricia; Gallacher, Eileen; Fraser, Paul J.; Langenfelds, Ray; Brenninkmeijer, Carl A. M.; Schwander, Jakob et al. (9 December 2016). "Tropospheric observations of CFC-114 and CFC-114a with a focus on long-term trends and emissions". Atmospheric Chemistry and Physics 16 (23): 15347–15358. doi:10.5194/acp-16-15347-2016. Bibcode: 2016ACP....1615347L.
- ↑ Suh, Dong Jin; Park, Tae-Jin; Lee, Byung-Gwon; Park, Kun-You (January 1996). "Synthesis of HFC-134a by isomerization and hydrogenation". Korean Journal of Chemical Engineering 13 (1): 75–81. doi:10.1007/BF02705892.
- ↑ 12.0 12.1 12.2 12.3 12.4 Davis, Maxine E.; Bernard, François; McGillen, Max R.; Fleming, Eric L.; Burkholder, James B. (1 July 2016). "UV and infrared absorption spectra, atmospheric lifetimes, and ozone depletion and global warming potentials for CCl<sub>2</sub>FCCl<sub>2</sub>F (CFC-112), CCl<sub>3</sub>CClF<sub>2</sub> (CFC-112a), CCl<sub>3</sub>CF<sub>3</sub> (CFC-113a), and CCl<sub>2</sub>FCF<sub>3</sub> (CFC-114a)". Atmospheric Chemistry and Physics 16 (12): 8043–8052. doi:10.5194/acp-16-8043-2016. Bibcode: 2016ACP....16.8043D.
- ↑ Western, Luke M. et al. (3 April 2023). "Global increase of ozone-depleting chlorofluorocarbons from 2010 to 2020". Nature Geoscience 16 (4): 309–313. doi:10.1038/s41561-023-01147-w. Bibcode: 2023NatGe..16..309W. https://research-information.bris.ac.uk/en/publications/9e103fef-e61c-49c7-a1a3-902540ec1d7c.
 |


