Chemistry:Hydrogenoxalate
| |
| Names | |
|---|---|
| IUPAC name
2-Hydroxy-2-oxoacetate[1]
| |
| Systematic IUPAC name
2-Hydroxy-2-oxoethanoate | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3601755 | |
| ChEBI | |
| 49515 | |
PubChem CID
|
|
| |
| |
| Properties | |
| HC 2O− 4 | |
| Molar mass | 89.026 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
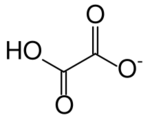
Hydrogenoxalate or hydrogen oxalate (IUPAC name: 2-Hydroxy-2-oxoacetate) is an anion with chemical formula HC
2O−
4 or HO–C(=O)–CO−
2, derived from oxalic acid by the loss of a single proton; or, alternatively, from the oxalate anion C
2O2−
4 by addition of a proton. The name is also used for any salt containing this anion. Especially in older literature, hydrogenoxalates may also be referred to as bioxalates, acid oxalates, or monobasic oxalates. Hydrogenoxalate is amphoteric, in that it can react both as an acid or a base.
Well characterized salts include sodium hydrogenoxalate (NaHC
2O
4),[2][3] potassium hydrogenoxalate (KHC
2O
4),[4] ammonium hydrogenoxalate ([NH
4]+
HC
2O−
4), rubidium hydrogenoxalate (RbHC
2O
4)[5] and dimethylammonium hydrogenoxalate ([(CH
3)
2NH]+
HC
2O−
4).[6]
Structure
Most hydrogenoxalate salts are hydrated. For example, potassium hydrogen oxalate crystallizes as 2KHC
2O
4 · H2O. These materials exhibit extended structures resulting from extensive hydrogen bonding and anion-cation interactions. The hydrates dehydrate upon heating:[4]
- 2KHC
2O
4 · H2O → 2 KHC
2O
4 + H
2O
Proton transfer in hydrogen oxalates has been studied.[7]
See also
References
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/3716971#section=IUPAC-Name&fullscreen=true
- ↑ Tellgren, Roland; Olovsson, Ivar (1971). "The crystal structures of normal and deuterated sodium hydrogen oxalate monohydrate NaHC2O4·H2O and NaDC2O4·D2O. Hydrogen bond studies XXXVI". The Journal of Chemical Physics 54: 127–134. doi:10.1063/1.1674582. Bibcode: 1971JChPh..54..127T.
- ↑ Delaplane, R. G.; Tellgren, R.; Olovsson, I. (1984). "Neutron diffraction study of sodium hydrogen oxalate monohydrate, NaHC2O4·H2O, at 120 K". Acta Crystallographica C40 (11): 1800–1803. doi:10.1107/S0108270184009616.
- ↑ 4.0 4.1 "The decomposition of potassium hydrogen oxalate hemihydrate". Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character (The Royal Society) 125 (799): 635–646. 1929. doi:10.1098/rspa.1929.0192. ISSN 0950-1207.
- ↑ Hamadène, M.; Kherfi, H.; Guehria-Laidoudi, A. (2006). "The polymeric anhydrous rubidium hydrogen oxalate". Acta Crystallographica A62: s280. doi:10.1107/S0108767306094414.
- ↑ Diallo, Waly; Gueye, Ndongo; Crochet, Aurélien; Plasseraud, Laurent; Cattey, Hélène (11 April 2015). "Crystal structure of dimethylammonium hydrogen oxalate hemi(oxalic acid)". Acta Crystallographica Section E: Crystallographic Communications (International Union of Crystallography (IUCr)) 71 (5): 473–475. doi:10.1107/s2056989015005964. ISSN 2056-9890.
- ↑ Bosch, Enric; Moreno, Miquel; Lluch, José María (1 January 1992). "The role of coupling in intramolecular proton transfer reactions. The hydrogen oxalate anion as an example". Canadian Journal of Chemistry (Canadian Science Publishing) 70 (1): 100–106. doi:10.1139/v92-017. ISSN 0008-4042.
 |

