Chemistry:Sodium hydrogenoxalate
| |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium 2-hydroxy-2-oxoacetate | |
Other names
| |
| Identifiers[2][1] | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
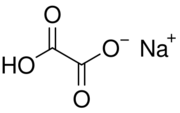
| NaHC 2O 4 | |
| Molar mass | 112.0167 g/mol[3] |
| Appearance | Colorless crystalline solid |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H312 | |
| P264, P270, P280, P301+312, P302+352, P312, P322, P330, P363, P501 | |
| Related compounds | |
Other anions
|
Sodium bicarbonate (oxalate replaced with carbonate) |
Other cations
|
|
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium hydrogenoxalate or sodium hydrogen oxalate is a chemical compound with the chemical formula NaHC
2O
4. It is an ionic compound. It is a sodium salt of oxalic acid H
2C
2O
4. It is an acidic salt, because it consists of sodium cations Na+
and hydrogen oxalate anions HC
2O−
4 or HO–C(=O)–CO−
2, in which only one acidic hydrogen atom in oxalic acid is replaced by sodium atom. The hydrogen oxalate anion can be described as the result of removing one hydrogen ion H+
from oxalic acid, or adding one to the oxalate anion C
2O2−
4.
Properties
Hydrates
The compound is commonly encountered as the anhydrous form or as the monohydrate NaHC
2O
4 · H2O. Both are colorless crystalline solids at ambient temperature.
The monohydrate can be obtained by evaporating a solution of the compound at room temperature.[4]
The crystal structure of NaHC
2O
4 · H2O is triclinic normal (pinacoidal, space group P1). The lattice parameters are a = 650.3 pm, b = 667.3 pm, c = 569.8 pm, α = 85.04°, β = 110.00°, γ = 105.02°, and Z = 2. The hydrogen oxalate ions are linked end to end in infinite chains by hydrogen bonds (257.1 pm). The chains are cross linked to form layers by both O–H···O bonds from the water molecules (280.8 pm, 282.6 pm) and by ionic bonds Na+
···O. These layers are in turn held together by Na+
···O bonds. The oxalate group is non-planar with an angle of twist about the C–C bond of 12.9°.[5]
Reactions
Upon being heated, sodium hydrogenoxalate converts to oxalic acid and sodium oxalate, the latter of which decomposes into sodium carbonate and carbon monoxide.[6]
- 2 NaHC
2O
4 → Na
2C
2O
4 + H
2C
2O
4
- Na
2C
2O
4 → Na
2CO
3 + CO
Toxicity
The health hazards posed by this compound are largely due to its acidity and to the toxic effects of oxalic acid and other oxalate or hydrogenoxalate salts, which can follow ingestion or absorption through the skin. The toxic effects include necrosis of tissues due to sequestration of calcium ions Ca2+, and the formation of poorly soluble calcium oxalate stones in the kidneys that can obstruct the kidney tubules.[2]
References
- ↑ 1.0 1.1 "2T9TH558WS: NaHC2O4". ChemSpider website, accessed on 2018-09-11
- ↑ 2.0 2.1 "Monosodium oxalate". NCBI PubChem website, accessed on 2018-09-11
- ↑ "Sodium Hydrogen Oxalate NaHC2O4". EndMemo.com website, accessed on 2018-09-11
- ↑ C. Ramki, R. Ezhil Vizhi (2017): "Growth, optical, electrical and mechanical properties of sodium hydrogen oxalate hydrate (NaHC2O4·H2O) single crystal for NLO applications". Materials Chemistry and Physics, volume 197, pages 70-78. doi:10.1016/j.matchemphys.2017.04.066
- ↑ Roland Tellgren and Ivar Olovsson (1971): "Hydrogen Bond Studies. XXXXVI. The Crystal Structures of Normal and Deuterated Sodium Hydrogen Oxalate Monohydrate NaHC2O4·H2O and NaDC2O4·D2O". Journal of Chemical Physics, volume 54, issue 1. doi:10.1063/1.1674582
- ↑ W. Balcerowiak; Cz. Latocha; J. Wasilewski (1980). "Thermoanalytical investigation of mixtures containing oxalic acid, sodium hydrogen oxalate and sodium oxalate". Journal of Thermal Analysis 18: 57–63. doi:10.1007/BF01909453.
 |

