Chemistry:3-Chlorobenzoic acid
From HandWiki
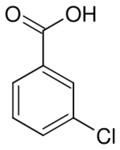
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Chlorobenzoic acid | |
| Other names
m-Chlorobenzoic acid
meta-Chlorobenzoic acid 3BZ | |
| Identifiers | |
3D model (JSmol)
|
|
| 907218 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 3664 | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H5ClO2 | |
| Molar mass | 156.57 g·mol−1 |
| Appearance | white solid |
| Density | 1.517 g/cm3 |
| Melting point | 154 °C (309 °F; 427 K) |
| Boiling point | 275 °C (527 °F; 548 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Flash point | 150 °C (302 °F; 423 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3-Chlorobenzoic acid is an organic compound with the molecular formula ClC6H4CO2H. It is a white solid that is soluble in some organic solvents and in aqueous base.[1]
Synthesis and occurrence
3-Chlorobenzoic acid is prepared by oxidation of 3-chlorotoluene.
It is a metabolic byproduct of the drug bupropion.[2]
References
- ↑ Takao Maki; Kazuo Takeda (2002). "Benzoic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_555. ISBN 3527306730..
- ↑ National Center for Biotechnology Information. "3-chlorobenzoic acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/3-chlorobenzoic_acid#section=Top.
 |

