Chemistry:Abamectin
 | |||
| |||
| Clinical data | |||
|---|---|---|---|
| Other names | Avermectin B1 (CAS name), MK-936 | ||
| ATCvet code | |||
| Legal status | |||
| Legal status |
| ||
| Identifiers | |||
| |||
| CAS Number | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEMBL | |||
| Chemical and physical data | |||
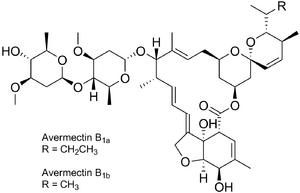
| Formula | C48H72O14 (B1a) C47H70O14 (B1b) | ||
| |||
| |||
| | |||
Abamectin (also called avermectin B1) is a widely used insecticide and anthelmintic. Abamectin, is a member of the avermectin family and is a natural fermentation product of soil dwelling[1] actinomycete Streptomyces avermitilis.[2] Abamectin differs from ivermectin, the popular member of the avermectin family, by a double bond between carbons 22 and 25.[2] Fermentation of Streptomyces avermitilis yields eight closely related avermectin homologs, with the B1a and B1b forms comprising the majority of the fermentation.[3] The non-proprietary name, abamectin, refers to a mixture of B1a (~80%) and B1b (~20%).[3] Out of all the avermectins, abamectin is the only one that is used both in agriculture and pharmaceuticals.[4]
Mode of Action
Avermectins bind to the glutamate-gated chloride channels that are found in invertebrate nerve and muscle cells.[5] They cause hyperpolarization of these cells resulting in paralysis and death.[5] Mammals only possess glutamate-gated chloride channels in the brain and spinal cord and as the avermectins have a low affinity for other mammalian ligand-gated channels and do not usually cross the blood–brain barrier, they are very safe for mammals.[6]
History
Avermectins were discovered in 1967 in fermentation broths of an actinomycete culture received from the Kitasato Institute in Japan, following an intensive search designed to find natural products with anthelmintic activity.[7] It was not until 1985 ivermectin was first used to treat infections with Onchocerca volvulus (onchocerciasis or river blindness) in humans by the United Nations.[8] The discoverers of avermectin, William C. Campbell and Satoshi Ōmura, shared the 2015 Nobel Prize in Physiology or Medicine.[9]
Activity
Abamectin is an insecticide as well as an acaricide (miticide)[2] and a nematicide. It is also used to control fire ants.[10] Abamectin is provided orally to horses for deworming them.[11]
Use
Abamectin is also used as a veterinary antihelmintic. Resistance to abamectin-based antihelmintics, although a growing problem, is not as common as to other classes of veterinary antihelmintics.[citation needed] The benzoate salt emamectin benzoate is also used as an insecticide. Avermectins have been used to treat various ailments caused by parasites in both humans and animals.[12] Avermectins including abamectin were studied for use as anti alcohol therapies.[13][12] Recently, ivermectin is being studied for use as an anti inflammatory agent.[14]
Environmental Fate
Abamectin degrades rapidly when exposed to light (photodegradation) on plant surfaces, in soil, dung and water.[15] Half life of Avermectins (including abamectin) varies between 0.5 and 23 days depending on the rate and substrate (water, soil, faeces or plant).[16] Avermectin B1a applied at 0.02-0.03 lb ai/acre (50% higher than recommended rates) resulted in very low residue.[17]
Non targets
Abamectin is highly toxic to bees either if they consume or come in direct contact.[18] However, plant parts exposed to abamectin spraying did not cause toxicity to bees 24 hours after treatment.[18][19] The reason for lower toxicity in foliage is due to a half life <24 hours in plant surfaces.[20]
Trade names
Trade names include Abba, Abathor, Affirm, Agri-Mek, Avid, Dynamec, Epi-Mek, Genesis Horse Wormer, Reaper, Termictine 5%, Vertimec, CAM-MEK 1.8% EC (cam for agrochemicals), Zephyr and Cure 1.8 EC.[citation needed]
References
- ↑ "Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis". Nature Biotechnology 21 (5): 526–531. May 2003. doi:10.1038/nbt820. PMID 12692562.
- ↑ 2.0 2.1 2.2 Ivermectin and Abamectin. Springer Science & Business Media. 6 December 2012. pp. 304–. ISBN 978-1-4612-3626-9. https://books.google.com/books?id=LAHaBwAAQBAJ&pg=PA304.
- ↑ 3.0 3.1 "Avermectins: Biochemical Mode of Action, Biological Activity and Agricultural Importance" (in en). Insecticides with Novel Modes of Action: Mechanisms and Application. Applied Agriculture. Berlin, Heidelberg: Springer. 1998. pp. 152–170. doi:10.1007/978-3-662-03565-8_9. ISBN 978-3-662-03565-8.
- ↑ "Structure and activity of avermectins and milbemycins in animal health". Veterinary Parasitology 59 (2): 139–156. September 1995. doi:10.1016/0304-4017(94)00743-V. PMID 7483237.
- ↑ 5.0 5.1 "Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics". Parasitology 131 Suppl (S1): S85–S95. 2006-03-29. doi:10.1017/S0031182005008218. PMID 16569295.
- ↑ "Ivermectin: panacea for resource-poor communities?". Trends in Parasitology 30 (9): 445–455. September 2014. doi:10.1016/j.pt.2014.07.005. PMID 25130507.
- ↑ "Avermectins, a novel class of compounds: implications for use in arthropod pest control". Annual Review of Entomology 36 (1): 91–117. 1991. doi:10.1146/annurev.en.36.010191.000515. PMID 2006872.
- ↑ "Ivermectin, 'wonder drug' from Japan: the human use perspective". Proceedings of the Japan Academy. Series B, Physical and Biological Sciences 87 (2): 13–28. 2011. doi:10.2183/pjab.87.13. PMID 21321478. Bibcode: 2011PJAB...87...13C.
- ↑ "The Nobel Prize in Physiology or Medicine 2015" (in en-US). https://www.nobelprize.org/prizes/medicine/2015/press-release/.
- ↑ "Ascend / Advance | Texas Imported Fire Ant Research and Management Project". https://fireant.tamu.edu/controlmethods/products/ascend_advance/.
- ↑ "Equine Megastore - Horse Wormers". https://www.equine-mega-store.com/horse-wormer.
- ↑ 12.0 12.1 "Avermectin Derivatives, Pharmacokinetics, Therapeutic and Toxic Dosages, Mechanism of Action, and Their Biological Effects". Pharmaceuticals 13 (8): 196. August 2020. doi:10.3390/ph13080196. PMID 32824399.
- ↑ "Multiday administration of ivermectin is effective in reducing alcohol intake in mice at doses shown to be safe in humans". NeuroReport 25 (13): 1018–1023. September 2014. doi:10.1097/wnr.0000000000000211. PMID 25004078.
- ↑ "Topical ivermectin improves allergic skin inflammation". Allergy 72 (8): 1212–1221. August 2017. doi:10.1111/all.13118. PMID 28052336.
- ↑ "Environmental effects of the usage of avermectins in livestock". Veterinary Parasitology 48 (1–4): 109–125. June 1993. doi:10.1016/0304-4017(93)90149-H. PMID 8346626.
- ↑ "Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin". Chemosphere 154: 204–214. July 2016. doi:10.1016/j.chemosphere.2016.03.113. PMID 27058912. Bibcode: 2016Chmsp.154..204B.
- ↑ "Residues of avermectin B1a in rotational crops and soils following soil treatment with [14C]avermectin B1a" (in en). Journal of Agricultural and Food Chemistry 35 (6): 859–864. 1987. doi:10.1021/jf00078a003. ISSN 0021-8561.
- ↑ 18.0 18.1 "Environmental Aspects of Abamectin Use in Crop Protection". Ivermectin and Abamectin. New York, NY: Springer New York. 1989. pp. 182–200. doi:10.1007/978-1-4612-3626-9_13. ISBN 978-1-4612-8184-9.
- ↑ "A review on the toxicity and non-target effects of macrocyclic lactones in terrestrial and aquatic environments". Current Pharmaceutical Biotechnology 13 (6): 1004–1060. May 2012. doi:10.2174/138920112800399257. PMID 22039795.
- ↑ "Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin". Chemosphere 154: 204–214. July 2016. doi:10.1016/j.chemosphere.2016.03.113. PMID 27058912. Bibcode: 2016Chmsp.154..204B.
Further reading
- "Pesticide Information Profile: Abamectin". Pesticide Management Education Program. Extension Toxicology Network (EXTOXNET). June 1996. http://extoxnet.orst.edu/pips/abamecti.htm.
- "Learn more about abamectin". Crop Protection Database. Farm Chemicals International. http://www.farmchemicalsinternational.com/cropprotection/cpd/?op=cpdproductdetail&pid=20440.
 |



