Chemistry:Ivermectin
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌaɪvərˈmɛktɪn/, EYE-vər-MEK-tin |
| Trade names | Stromectol, Soolantra, Sklice, others |
| Other names | MK-933 |
| AHFS/Drugs.com | |
| MedlinePlus | a607069 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | not determined |
| Protein binding | 93% |
| Metabolism | Liver (CYP450) |
| Elimination half-life | 18 hours |
| Excretion | Feces; <1% urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C48H74O14 (22,23-dihydroavermectin B1a) C47H72O14 (22,23-dihydroavermectin B1b) |
| Molar mass |
|
| |
| |
| | |
Ivermectin is an antiparasitic drug.[5] After its discovery in 1975,[6] its first uses were in veterinary medicine to prevent and treat heartworm and acariasis.[7] Approved for human use in 1987,[8] it is used to treat infestations including head lice, scabies, river blindness (onchocerciasis), strongyloidiasis, trichuriasis, ascariasis and lymphatic filariasis.[7][9][10][11] It works through many mechanisms to kill the targeted parasites,[9] and can be taken by mouth, or applied to the skin for external infestations.[9][12] It belongs to the avermectin family of medications.[9]
William Campbell and Satoshi Ōmura won the 2015 Nobel Prize in Physiology or Medicine for its discovery and applications.[13] It is on the World Health Organization's List of Essential Medicines,[14][15] and is approved by the U.S. Food and Drug Administration as an antiparasitic agent.[16] In 2020, it was the 423rd most commonly prescribed medication in the United States, with more than 100,000 prescriptions.[17] It is available as a generic medicine.[18][19]
During the COVID-19 pandemic, misinformation has been widely spread claiming that ivermectin is beneficial for treating and preventing COVID-19.[20][21] Such claims are not backed by credible scientific evidence.[22][23][24] Multiple major health organizations, including the U.S. Food and Drug Administration,[25] the U.S. Centers for Disease Control and Prevention,[26] the European Medicines Agency,[27] and the World Health Organization have stated that ivermectin is not authorized or approved to treat COVID-19.[23][28]
Medical uses
Ivermectin is used to treat human diseases caused by roundworms and a wide variety of external parasites.[29]
Worm infections
For river blindness (onchocerciasis) and lymphatic filariasis, ivermectin is typically given as part of mass drug administration campaigns that distribute the drug to all members of a community affected by the disease.[30] Adult worms survive in the skin and eventually recover to produce larval worms again; to keep the worms at bay, ivermectin is given at least once per year for the 10–15-year lifespan of the adult worms.[31]
The World Health Organization (WHO) considers ivermectin the drug of choice for strongyloidiasis.[32] Ivermectin is also the primary treatment for Mansonella ozzardi and cutaneous larva migrans.[33][34] The U.S. Centers for Disease Control and Prevention (CDC) recommends ivermectin, albendazole, or mebendazole as treatments for ascariasis.[35][note 1] Ivermectin is sometimes added to albendazole or mebendazole for whipworm treatment, and is considered a second-line treatment for gnathostomiasis.[34][39]
Mites and insects
Ivermectin is also used to treat infection with parasitic arthropods. Scabies – infestation with the mite Sarcoptes scabiei – is most commonly treated with topical permethrin or oral ivermectin. A single application of permethrin is more efficacious than a single treatment of ivermectin. For most scabies cases, ivermectin is used in a two dose regimen: a first dose kills the active mites, but not their eggs. Over the next week, the eggs hatch, and a second dose kills the newly hatched mites.[40][41] The two dose regimen of ivermectin has similar efficacy to the single dose permethrin treatment. Ivermectin is, however, more effective than permethrin when used in the mass treatment of endemic scabies.[42]
For severe "crusted scabies", where the parasite burden is orders of magnitude higher than usual, the U.S. Centers for Disease Control and Prevention (CDC) recommends up to seven doses of ivermectin over the course of a month, along with a topical antiparasitic.[41] Both head lice and pubic lice can be treated with oral ivermectin, an ivermectin lotion applied directly to the affected area, or various other insecticides.[43][44] Ivermectin is also used to treat rosacea and blepharitis, both of which can be caused or exacerbated by Demodex folliculorum mites.[45][46]
Contraindications
The only absolute contraindication to the use of ivermectin is hypersensitivity to the active ingredient or any component of the formulation.[47][48] In children under the age of five or those who weigh less than 15 kilograms (33 pounds),[49] there is limited data regarding the efficacy or safety of ivermectin, though the available data demonstrate few adverse effects.[50] However, the American Academy of Pediatrics cautions against use of ivermectin in such patients, as the blood-brain barrier is less developed, and thus there may be an increased risk of particular CNS side effects such as encephalopathy, ataxia, coma, or death.[51] The American Academy of Family Physicians also recommends against use in these patients, given a lack of sufficient data to prove drug safety.[52] Ivermectin is secreted in very low concentration in breast milk.[53] It remains unclear if ivermectin is safe during pregnancy.[54]
Adverse effects
Side effects, although uncommon, include fever, itching, and skin rash when taken by mouth;[9] and red eyes, dry skin, and burning skin when used topically for head lice.[55] It is unclear if the drug is safe for use during pregnancy, but it is probably acceptable for use during breastfeeding.[56]
Ivermectin is considered relatively free of toxicity in standard doses (around 300 µg/kg).[57][58] Based on the data drug safety sheet for ivermectin,[lower-alpha 1] side effects are uncommon. However, serious adverse events following ivermectin treatment are more common in people with very high burdens of larval Loa loa worms in their blood.[59] Those who have over 30,000 microfilaria per milliliter of blood risk inflammation and capillary blockage due to the rapid death of the microfilaria following ivermectin treatment.[59]
One concern is neurotoxicity after large overdoses, which in most mammalian species may manifest as central nervous system depression,[60] ataxia, coma, and even death,[61][62] as might be expected from potentiation of inhibitory chloride channels.[63]
Since drugs that inhibit the enzyme CYP3A4 often also inhibit P-glycoprotein transport, the risk of increased absorption past the blood-brain barrier exists when ivermectin is administered along with other CYP3A4 inhibitors. These drugs include statins, HIV protease inhibitors, many calcium channel blockers, lidocaine, the benzodiazepines, and glucocorticoids such as dexamethasone.[64]
During the course of a typical treatment, ivermectin can cause minor aminotransferase elevations. In rare cases it can cause mild clinically apparent liver disease.[65]
To provide context for the dosing and toxicity ranges, the -1">50 of ivermectin in mice is 25 mg/kg (oral), and 80 mg/kg in dogs, corresponding to an approximated human-equivalent dose LD50 range of 2.02–43.24 mg/kg,[66] which is far in excess of its FDA-approved usage (a single dose of 0.150–0.200 mg/kg to be used for specific parasitic infections).[2] While ivermectin has also been studied for use in COVID-19, and while it has some ability to inhibit SARS-CoV-2 in vitro, achieving 50% inhibition in vitro was found to require an estimated oral dose of 7.0 mg/kg (or 35x the maximum FDA-approved dosage),[67] high enough to be considered ivermectin poisoning.[66] Despite insufficient data to show any safe and effective dosing regimen for ivermectin in COVID-19, doses have been taken far in excess of FDA-approved dosing, leading the CDC to issue a warning of overdose symptoms including nausea, vomiting, diarrhea, hypotension, decreased level of consciousness, confusion, blurred vision, visual hallucinations, loss of coordination and balance, seizures, coma, and death. The CDC advises against consuming doses intended for livestock or doses intended for external use and warns that increasing misuse of ivermectin-containing products is resulting in an increase in harmful overdoses.[68]
Pharmacology

Mechanism of action
Ivermectin and its related drugs act by interfering with the nerve and muscle functions of helminths and insects.[69] The drug binds to glutamate-gated chloride channels common to invertebrate nerve and muscle cells.[70] The binding pushes the channels open, which increases the flow of chloride ions and hyper-polarizes the cell membranes,[69] paralyzing and killing the invertebrate.[70] Ivermectin is safe for mammals (at the normal therapeutic doses used to cure parasite infections) because mammalian glutamate-gated chloride channels only occur in the brain and spinal cord: the causative avermectins usually do not cross the blood–brain barrier, and are unlikely to bind to other mammalian ligand-gated channels.[70]
Pharmacokinetics
Ivermectin can be given by mouth, topically, or via injection. Oral doses are absorbed into systemic circulation; the alcoholic solution form is more orally available than tablet and capsule forms. Ivermectin is widely distributed in the body.[71]
Ivermectin does not readily cross the blood–brain barrier of mammals due to the presence of P-glycoprotein (the MDR1 gene mutation affects the function of this protein).[72] Crossing may still become significant if ivermectin is given at high doses, in which case brain levels peak 2–5 hours after administration. In contrast to mammals, ivermectin can cross the blood–brain barrier in tortoises, often with fatal consequences.[73]
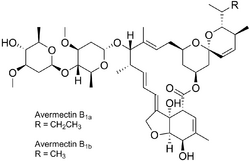
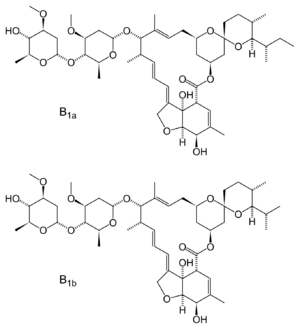
Chemistry
Fermentation of Streptomyces avermitilis yields eight closely related avermectin homologues, of which B1a and B1b form the bulk of the products isolated. In a separate chemical step, the mixture is hydrogenated to give ivermectin, which is an approximately 80:20 mixture of the two 22,23-dihydroavermectin compounds.[74][75][5]
Ivermectin is a macrocyclical lactone.[76]
History
The avermectin family of compounds was discovered by Satoshi Ōmura of Kitasato University and William Campbell of Merck.[5] In 1970, Ōmura isolated a strain of Streptomyces avermitilis from woodland soil near a golf course along the south east coast of Honshu, Japan.[5] Ōmura sent the bacteria to William Campbell, who showed that the bacterial culture could cure mice infected with the roundworm Heligmosomoides polygyrus.[5] Campbell isolated the active compounds from the bacterial culture, naming them "avermectins" and the bacterium Streptomyces avermitilis for the compounds' ability to clear mice of worms (in Latin: a 'without', vermis 'worms').[5] Of the various avermectins, Campbell's group found the compound "avermectin B1" to be the most potent when taken orally.[5] They synthesized modified forms of avermectin B1 to improve its pharmaceutical properties, eventually choosing a mixture of at least 80% 22,23-dihydroavermectin B1a and up to 20% 22,23-dihydroavermectin B1b, a combination they called "ivermectin".[5][77]
The discovery of ivermectin has been described as a combination of "chance and choice." Merck was looking for a broad-spectrum anthelmintic, which ivermectin is indeed; however, Campbell noted that they "...also found a broad-spectrum agent for the control of ectoparasitic insects and mites."[78]
Merck began marketing ivermectin as a veterinary antiparasitic in 1981.[5] By 1986, ivermectin was registered for use in 46 countries and was administered massively to cattle, sheep and other animals.[79] By the late 1980s, ivermectin was the bestselling veterinary medicine in the world.[5] Following its blockbuster success as a veterinary antiparasitic, another Merck scientist, Mohamed Aziz, collaborated with the World Health Organization to test the safety and efficacy of ivermectin against onchocerciasis in humans.[8] They found it to be highly safe and effective,[80] triggering Merck to register ivermectin for human use as "Mectizan" in France in 1987.[8] A year later, Merck CEO Roy Vagelos agreed that Merck would donate all ivermectin needed to eradicate river blindness.[8] In 1998, that donation would be expanded to include ivermectin used to treat lymphatic filariasis.[8]
Ivermectin earned the title of "wonder drug" for the treatment of nematodes and arthropod parasites.[81] Ivermectin has been used safely by hundreds of millions of people to treat river blindness and lymphatic filariasis.[5]
Half of the 2015 Nobel Prize in Physiology or Medicine was awarded jointly to Campbell and Ōmura for discovering avermectin, "the derivatives of which have radically lowered the incidence of river blindness and lymphatic filariasis, as well as showing efficacy against an expanding number of other parasitic diseases".[13][82]
Society and culture
COVID-19 misinformation
Economics
The initial price proposed by Merck in 1987 was US$6 per treatment, which was unaffordable for patients who most needed ivermectin.[83] The company donated hundreds of millions of courses of treatments since 1988 in more than 30 countries.[83] Between 1995 and 2010, using donated ivermectin to prevent river blindness, the program is estimated to have prevented seven million years of disability at a cost of US$257,000,000.[84]
Ivermectin is considered an inexpensive drug.[85] As of 2019, ivermectin tablets (Stromectol) in the United States were the least expensive treatment option for lice in children at approximately US$9.3, while Sklice, an ivermectin lotion, cost around US$300 for 120 mL (4 US fl oz).[86]
(As of 2019), the cost effectiveness of treating scabies and lice with ivermectin has not been studied.[87][88]
Brand names
It is sold under the brand names Heartgard, Sklice[89] and Stromectol[2] in the United States, Ivomec worldwide by Merial Animal Health, Mectizan in Canada by Merck, Iver-DT[90] in Nepal by Alive Pharmaceutical and Ivexterm in Mexico by Valeant Pharmaceuticals International. In Southeast Asian countries, it is marketed by Delta Pharma Ltd. under the trade name Scabo 6. The formulation for rosacea treatment is sold under the brand name Soolantra.[3] While in development, it was assigned the code MK-933 by Merck.[91]
Research
Parasitic disease
Ivermectin has been researched in laboratory animals, as a potential treatment for trichinosis[30] and trypanosomiasis.[92]
Tropical diseases
(As of 2016) ivermectin was studied as a potential antiviral agent against chikungunya and yellow fever.[93] In chikungunya, ivermectin showed a wide in vitro safety margin as an antiviral.[93]
Ivermectin is also of interest in the prevention of malaria, as it is toxic to both the malaria plasmodium itself and the mosquitos that carry it.[94][95] A direct effect on malaria parasites could not be shown in an experimental infection of volunteers with Plasmodium falciparum.[96] Use of ivermectin at higher doses necessary to control malaria is probably safe, though large clinical trials have not yet been done to definitively establish the efficacy or safety of ivermectin for prophylaxis or treatment of malaria.[97][57] Mass drug administration of a population with ivermectin to treat and prevent nematode infestation is effective for eliminating malaria-bearing mosquitos and thereby potentially reducing infection with residual malaria parasites.[98] Whilst effective in killing malaria-bearing mosquitos, a 2021 Cochrane review found that, to date, the evidence shows no significant impact on reducing incidence of malaria transmission from the community administration of ivermectin.[97]
One alternative to ivermectin is moxidectin, which has been approved by the Food and Drug Administration for use in people with river blindness.[99] Moxidectin has a longer half-life than ivermectin and may eventually supplant ivermectin as it is a more potent microfilaricide, but there is a need for additional clinical trials, with long-term follow-up, to assess whether moxidectin is safe and effective for treatment of nematode infection in children and women of childbearing potential.[100][101]
There is tentative evidence that ivermectin kills bedbugs, as part of integrated pest management for bedbug infestations.[102][103][104] However, such use may require a prolonged course of treatment which is of unclear safety.[105]
NAFLD
In 2013, ivermectin was demonstrated as a novel ligand of the farnesoid X receptor,[106][107] a therapeutic target for nonalcoholic fatty liver disease.[108]
COVID-19
During the COVID-19 pandemic, ivermectin was researched for possible utility in preventing and treating COVID-19, but no good evidence of benefit was found.[109][110]
Veterinary use
Ivermectin is routinely used to control parasitic worms in the gastrointestinal tract of ruminant animals. These parasites normally enter the animal when it is grazing, pass the bowel, and set and mature in the intestines, after which they produce eggs that leave the animal via its droppings and can infest new pastures. Ivermectin is only effective in killing some of these parasites, this is because of an increase in anthelmintic resistance.[111] This resistance has arisen from the persistent use of the same anthelmintic drugs for the past 40 years.[112][113]
In dogs, ivermectin is routinely used as prophylaxis against heartworm.[114] Dogs with defects in the P-glycoprotein gene (MDR1), often collie-like herding dogs, can be severely poisoned by ivermectin. The mnemonic "white feet, don't treat" refers to Scotch collies that are vulnerable to ivermectin.[115] Some other dog breeds (especially the Rough Collie, the Smooth Collie, the Shetland Sheepdog, and the Australian Shepherd), also have a high incidence of mutation within the MDR1 gene (coding for P-glycoprotein) and are sensitive to the toxic effects of ivermectin.[116][117] Clinical evidence suggests kittens are susceptible to ivermectin toxicity.[118] A 0.01% ivermectin topical preparation for treating ear mites in cats is available.[119]
Ivermectin is sometimes used as an acaricide in reptiles, both by injection and as a diluted spray. While this works well in some cases, care must be taken, as several species of reptiles are very sensitive to ivermectin. Use in turtles is particularly contraindicated.[120]
A characteristic of the antinematodal action of ivermectin is its potency: for instance, to combat Dirofilaria immitis in dogs, ivermectin is effective at 0.001 milligram per kilogram of body weight when administered orally.[77]
For dogs, the insecticide spinosad may have the effect of increasing the toxicity of ivermectin.[121][122]
Notes
- ↑ This recommendation is not universal. The World Health Organization recommends ascariasis be treated with mebendazole or pyrantel pamoate,[36] while the textbook Parasitic Diseases recommends albendazole or mebendazole.[37] A 2020 Cochrane review concluded that the three drugs are equally safe and effective for treating ascariasis.[38]
- ↑ New Drug Application Identifier: 50-742/S-022
References
- ↑ "Search Page – Drug and Health Product Register". October 23, 2014. https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00498.
- ↑ 2.0 2.1 2.2 "Stromectol – ivermectin tablet". December 15, 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=681888c9-af79-4b7d-ae80-c3f4f6f1effd.
- ↑ 3.0 3.1 "Soolantra – ivermectin cream". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b1d5b166-ab06-4ab5-b0c6-31126238118a.
- ↑ "List of nationally authorised medicinal products". European Medicines Agency. November 26, 2020. https://www.ema.europa.eu/documents/psusa/ivermectin-topical-use-list-nationally-authorised-medicinal-products-psusa/00010376/202004_en.pdf.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 "Ivermectin – Old Drug, New Tricks?". Trends in Parasitology 33 (6): 463–472. June 2017. doi:10.1016/j.pt.2017.02.004. PMID 28285851.
- ↑ "History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents". Current Pharmaceutical Biotechnology 13 (6): 853–865. May 2012. doi:10.2174/138920112800399095. PMID 22039784.
- ↑ 7.0 7.1 Saunders Handbook of Veterinary Drugs: Small and Large Animal (4 ed.). Elsevier Health Sciences. 2015. p. 420. ISBN 978-0-323-24486-2. https://books.google.com/books?id=SJvmCgAAQBAJ&pg=PA420.
- ↑ 8.0 8.1 8.2 8.3 8.4 "Reflections on the Nobel Prize for Medicine 2015—The Public Health Legacy and Impact of Avermectin and Artemisinin". Trends in Parasitology 31 (12): 605–607. December 2015. doi:10.1016/j.pt.2015.10.008. PMID 26552892.
- ↑ 9.0 9.1 9.2 9.3 9.4 "Ivermectin". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/ivermectin.html.
- ↑ Drug Discovery a History. Chichester: John Wiley & Sons. 2005. p. 333. ISBN 978-0-470-01552-0. https://books.google.com/books?id=jglFsz5EJR8C&pg=PA333. Retrieved April 5, 2020.
- ↑ "Ascariasis – Resources for Health Professionals". U.S. Centers for Disease Control and Prevention (CDC). August 23, 2019. https://www.cdc.gov/parasites/ascariasis/health_professionals/index.html.
- ↑ "The efficacy of topical and oral ivermectin in the treatment of human scabies". Annals of Parasitology 61 (1): 11–16. 2015. PMID 25911032. https://www.annals-parasitology.eu/go.live.php/download_default/D660/2015-61-1_11.pdf.
- ↑ 13.0 13.1 "The Nobel Prize in Physiology or Medicine 2015". Nobel Foundation. https://www.nobelprize.org/nobel_prizes/medicine/laureates/2015/press.pdf.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness". International Journal of Infectious Diseases 103: 214–216. February 2021. doi:10.1016/j.ijid.2020.11.191. PMID 33278625.
- ↑ "Ivermectin - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Ivermectin.
- ↑ "Ivermectin: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=204154.
- ↑ "Ivermectin lotion: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=210720.
- ↑ "Anatomy of a conspiracy theory: how misinformation travels on Facebook". The Guardian. https://www.theguardian.com/australia-news/ng-interactive/2021/mar/11/anatomy-of-a-conspiracy-theory-how-misinformation-travels-on-facebook.
- ↑ "Fact-checking claim about the use of ivermectin to treat COVID-19". PolitiFact (Washington, DC). https://www.politifact.com/factchecks/2021/apr/23/instagram-posts/fact-checking-claim-about-use-ivermectin-treat-cov/.
- ↑ "Ivermectin for preventing and treating COVID-19". The Cochrane Database of Systematic Reviews 2022 (6): CD015017. June 2022. doi:10.1002/14651858.CD015017.pub3. PMID 35726131.
- ↑ 23.0 23.1 "EMA advises against use of ivermectin for the prevention or treatment of COVID-19 outside randomised clinical trials". European Medicines Agency. March 22, 2021. https://www.ema.europa.eu/en/news/ema-advises-against-use-ivermectin-prevention-treatment-covid-19-outside-randomised-clinical-trials.
- ↑ "Misleading clinical evidence and systematic reviews on ivermectin for COVID-19". BMJ Evidence-Based Medicine 27 (3): 156–158. April 2021. doi:10.1136/bmjebm-2021-111678. PMID 33888547.
- ↑ "Why You Should Not Use Ivermectin to Treat or Prevent COVID-19". December 10, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19.
- ↑ "Rapid Increase in Ivermectin Prescriptions and Reports of Severe Illness Associated with Use of Products Containing Ivermectin to Prevent or Treat COVID-19". CDC Health Alert Network CDCHAN-00449. August 26, 2021. https://emergency.cdc.gov/han/2021/pdf/CDC_HAN_449.pdf. Retrieved January 4, 2022.
- ↑ "EMA advises against use of ivermectin for the prevention or treatment of COVID-19 outside randomised clinical trials". European Medicines Agency. March 22, 2021. https://www.ema.europa.eu/en/news/ema-advises-against-use-ivermectin-prevention-treatment-covid-19-outside-randomised-clinical-trials.
- ↑ "WHO advises that ivermectin only be used to treat COVID-19 within clinical trials". https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials.
- ↑ "Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations". The Journal of Antibiotics 70 (5): 495–505. May 2017. doi:10.1038/ja.2017.11. PMID 28196978. ""Ivermectin was a revelation. It had a broad spectrum of activity, was highly efficacious, acting robustly at low doses against a wide variety of nematode, insect and acarine parasites. It proved to be extremely effective against most common intestinal worms (except tapeworms), could be administered orally, topically or parentally and showed no signs of cross-resistance with other commonly used anti-parasitic compounds."".
- ↑ 30.0 30.1 "Ivermectin: From theory to clinical application". International Journal of Antimicrobial Agents 54 (2): 134–142. August 2019. doi:10.1016/j.ijantimicag.2019.05.003. PMID 31071469.
- ↑ "Onchocerciasis". World Health Organization. June 14, 2019. https://www.who.int/news-room/fact-sheets/detail/onchocerciasis.
- ↑ "Strongyloidiasis". World Health Organization. https://www.who.int/intestinal_worms/epidemiology/strongyloidiasis/en/.
- ↑ "26. Other Nematodes of Medical Importance". Parasitic Diseases (7 ed.). New York: Parasites Without Borders. 2019. p. 294. https://parasiteswithoutborders.com/wp-content/uploads/2020/02/PD7thEditionLowResVersion5-11-2019.pdf. Retrieved January 26, 2021.
- ↑ 34.0 34.1 "27. Aberrant Nematode Infections". Parasitic Diseases (7 ed.). New York: Parasites Without Borders. 2019. p. 299. https://parasiteswithoutborders.com/wp-content/uploads/2020/02/PD7thEditionLowResVersion5-11-2019.pdf. Retrieved January 26, 2021.
- ↑ "Ascariasis – Resources for Health Professionals". U.S. Centers for Disease Control and Prevention (CDC). May 20, 2020. https://www.cdc.gov/parasites/ascariasis/health_professionals/index.html#tx.
- ↑ "Water related diseases – Ascariasis". World Health Organization. https://www.who.int/water_sanitation_health/diseases-risks/diseases/ascariasis/en/.
- ↑ "18. Ascaris lumbricoides". Parasitic Diseases (7 ed.). New York: Parasites Without Borders. 2019. p. 211. https://parasiteswithoutborders.com/wp-content/uploads/2020/02/PD7thEditionLowResVersion5-11-2019.pdf. Retrieved January 26, 2021.
- ↑ "Anthelmintic drugs for treating ascariasis". The Cochrane Database of Systematic Reviews 2020 (4): CD010599. April 2020. doi:10.1002/14651858.CD010599.pub2. PMID 32289194.
- ↑ "17. Trichuris trichiura". Parasitic Diseases (7 ed.). New York: Parasites Without Borders. 2019. p. 201. https://parasiteswithoutborders.com/wp-content/uploads/2020/02/PD7thEditionLowResVersion5-11-2019.pdf. Retrieved January 26, 2021.
- ↑ "Ectoparasites: Scabies". Journal of the American Academy of Dermatology 82 (3): 533–548. March 2020. doi:10.1016/j.jaad.2019.05.109. PMID 31310840.
- ↑ 41.0 41.1 "Scabies – Medications". U.S. Centers for Disease Control and Prevention (CDC). October 2, 2019. https://www.cdc.gov/parasites/scabies/health_professionals/meds.html.
- ↑ The Itch: Scabies (1st ed.). United Kingdom: Oxford University Press. 2022. pp. 146–152. ISBN 978-0-19-284840-6.
- ↑ "Lice and Scabies: Treatment Update". American Family Physician 99 (10): 635–642. May 2019. PMID 31083883.
- ↑ "Pubic "Crab" Lice – Treatment". U.S. Centers for Disease Control and Prevention (CDC). September 12, 2019. https://www.cdc.gov/parasites/lice/pubic/treatment.html.
- ↑ "Rosacea". The New England Journal of Medicine 377 (18): 1754–1764. November 2017. doi:10.1056/NEJMcp1506630. PMID 29091565.
- ↑ "Demodex mites". Clinics in Dermatology 32 (6): 739–743. 2014. doi:10.1016/j.clindermatol.2014.02.012. PMID 25441466.
- ↑ "Ivermectin (PIM 292)". InChem. https://inchem.org/documents/pims/pharm/ivermect.htm.
- ↑ "Stromectol (ivermectin) dose, indications, adverse effects, interactions". Prescribers' Digital Reference. https://www.pdr.net/drug-summary/Stromectol-ivermectin-391.
- ↑ "Ivermectin: pharmacology and application in dermatology". International Journal of Dermatology 44 (12): 981–988. December 2005. doi:10.1111/j.1365-4632.2004.02253.x. PMID 16409259. https://www.academia.edu/33924249. Retrieved April 6, 2020.
- ↑ "Question 1: Is it safe to use ivermectin in children less than five years of age and weighing less than 15 kg?". Archives of Disease in Childhood 103 (5): 514–519. May 2018. doi:10.1136/archdischild-2017-314505. PMID 29463522.
- ↑ "Ivermectin – Drug Monographs – Pediatric Care Online". American Academy of Pediatrics Drug Monographs. August 2021. https://publications.aap.org/pediatriccare/drug-monograph/18/6030. Retrieved April 2, 2022.
- ↑ "Ivermectin Use in Scabies". American Family Physician 68 (6): 1089–1092. September 15, 2003. ISSN 0002-838X. PMID 14524395. https://www.aafp.org/afp/2003/0915/p1089.html. Retrieved April 2, 2022.
- ↑ "New changes in pregnancy and lactation labelling: Review of dermatologic drugs". International Journal of Women's Dermatology 5 (4): 216–226. September 2019. doi:10.1016/j.ijwd.2019.05.002. PMID 31700976.
- ↑ "Safety of oral ivermectin during pregnancy: a systematic review and meta-analysis". The Lancet. Global Health 8 (1): e92–e100. January 2020. doi:10.1016/S2214-109X(19)30453-X. PMID 31839144.
- ↑ "Ivermectin (topical)". The American Society of Health-System Pharmacists. July 27, 2020. https://www.drugs.com/monograph/ivermectin-topical.html.
- ↑ "Ivermectin Levels and Effects while Breastfeeding". https://www.drugs.com/breastfeeding/ivermectin.html.
- ↑ 57.0 57.1 "Safety of high-dose ivermectin: a systematic review and meta-analysis". The Journal of Antimicrobial Chemotherapy 75 (4): 827–834. April 2020. doi:10.1093/jac/dkz524. PMID 31960060.
- ↑ "Ivermectin: An Anthelmintic, an Insecticide, and Much More". Trends in Parasitology 37 (1): 48–64. January 2021. doi:10.1016/j.pt.2020.10.005. PMID 33189582.
- ↑ 59.0 59.1 "Effect of a Single Standard Dose (150–200 μg/kg) of Ivermectin on Loa loa Microfilaremia: Systematic Review and Meta-analysis". Open Forum Infectious Diseases 6 (4): ofz019. April 2019. doi:10.1093/ofid/ofz019. PMID 30968052.
- ↑ "Ivermectin: An Anthelmintic, an Insecticide, and Much More". Trends in Parasitology 37 (1): 48–64. January 2021. doi:10.1016/j.pt.2020.10.005. PMID 33189582. ""Although relatively free from toxicity, ivermectin – when large overdoses are administered – may cross the blood–brain barrier, producing depressant effects on the CNS"".
- ↑ "Serious adverse reactions associated with ivermectin: A systematic pharmacovigilance study in sub-Saharan Africa and in the rest of the World". PLOS Neglected Tropical Diseases 15 (4): e0009354. April 2021. doi:10.1371/journal.pntd.0009354. PMID 33878105. ""Few hours after administration: nausea, vomiting, abdominal pain, salivation, tachycardia, hypotension, ataxia, pyramidal signs, binocular diplopia"".
- ↑ Office of the Commissioner (March 12, 2021). "Why You Should Not Use Ivermectin to Treat or Prevent COVID-19". https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19. ""You can also overdose on ivermectin, which can cause nausea, vomiting, diarrhea, hypotension (low blood pressure), allergic reactions (itching and hives), dizziness, ataxia (problems with balance), seizures, coma and even death.""
- ↑ "Avermectin Derivatives, Pharmacokinetics, Therapeutic and Toxic Dosages, Mechanism of Action, and Their Biological Effects". Pharmaceuticals 13 (8): 196. August 2020. doi:10.3390/ph13080196. PMID 32824399. ""Based on the reported neurotoxicity and metabolic pathway of IVM, caution should be taken to conduct clinical trial on its antiviral potentials. The GABA-gated chloride channels in the human nervous system might be a target for IVM, this is because the BBB in disease-patient might be a weakened as a result of inflammation and other destructive processes, allowing IVM to cross the BBB and gain access to the CNS where it can elicit its neurotoxic effect"".
- ↑ Goodman & Gilman's The Pharmacological Basis of Therapeutics. (11th ed.). New York: McGraw-Hill. 2006. pp. 1084–87. ISBN 978-0-07-142280-2. OCLC 1037399847.
- ↑ "Ivermectin". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, Maryland: National Institute of Diabetes and Digestive and Kidney Diseases. 2012. https://www.ncbi.nlm.nih.gov/books/NBK548921/. Retrieved May 30, 2021.
- ↑ 66.0 66.1 "The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug". American Journal of Cancer Research 8 (2): 317–331. 2018. PMID 29511601.
- ↑ "The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19". Clinical Pharmacology and Therapeutics 108 (4): 762–765. October 2020. doi:10.1002/cpt.1889. PMID 32378737.
- ↑ "Rapid Increase in Ivermectin Prescriptions and Reports of Severe Illness Associated with Use of Products Containing Ivermectin to Prevent or Treat COVID-19". August 26, 2021. https://emergency.cdc.gov/han/2021/han00449.asp.
- ↑ 69.0 69.1 "Ivermectin: An Anthelmintic, an Insecticide, and Much More". Trends in Parasitology 37 (1): 48–64. January 2021. doi:10.1016/j.pt.2020.10.005. PMID 33189582.
- ↑ 70.0 70.1 70.2 "Ivermectin: panacea for resource-poor communities?". Trends in Parasitology 30 (9): 445–455. September 2014. doi:10.1016/j.pt.2014.07.005. PMID 25130507.
- ↑ "The pharmacokinetics and interactions of ivermectin in humans—a mini-review". The AAPS Journal 10 (1): 42–46. 2008. doi:10.1208/s12248-007-9000-9. PMID 18446504.
- ↑ "What have we learnt thus far from mice with disrupted P-glycoprotein genes?". European Journal of Cancer 32A (6): 985–990. June 1996. doi:10.1016/0959-8049(96)00063-9. PMID 8763339.
- ↑ "Toxicity and efficacy of ivermectin in chelonians". Journal of the American Veterinary Medical Association 183 (11): 1195–1197. December 1983. PMID 6689009. https://repository.si.edu/bitstream/handle/10088/4426/Teare1983.pdf. Retrieved October 26, 2021.
- ↑ "Avermectins, a novel class of compounds: implications for use in arthropod pest control". Annual Review of Entomology 36: 91–117. 1991. doi:10.1146/annurev.en.36.010191.000515. PMID 2006872.
- ↑ "Avermectins: Biochemical Mode of Action, Biological Activity and Agricultural Importance". Insecticides with Novel Modes of Action. Applied Agriculture. Berlin, Heidelberg: Springer. 1998. pp. 152–70. doi:10.1007/978-3-662-03565-8_9. ISBN 978-3-642-08314-3.
- ↑ "Ivermectin: an update". Parasitology Today 1 (1): 10–16. July 1985. doi:10.1016/0169-4758(85)90100-0. PMID 15275618.
- ↑ 77.0 77.1 "Ivermectin: a potent new antiparasitic agent". Science 221 (4613): 823–828. August 1983. doi:10.1126/science.6308762. PMID 6308762. Bibcode: 1983Sci...221..823C.
- ↑ "Serendipity and new drugs for infectious disease". ILAR Journal 46 (4): 352–356. January 1, 2005. doi:10.1093/ilar.46.4.352. PMID 16179743.
- ↑ "The life and times of ivermectin – a success story". Nature Reviews. Microbiology 2 (12): 984–989. December 2004. doi:10.1038/nrmicro1048. PMID 15550944.
- ↑ "The onchocerciasis chronicle: from the beginning to the end?". Trends in Parasitology 28 (7): 280–288. July 2012. doi:10.1016/j.pt.2012.04.005. PMID 22633470.
- ↑ "Ivermectin 20 years on: maturation of a wonder drug". Trends in Parasitology 21 (11): 530–532. November 2005. doi:10.1016/j.pt.2005.08.014. PMID 16126457.
- ↑ "The Nobel Prize in Physiology or Medicine 2015". 18 March 2023. https://www.nobelprize.org/prizes/medicine/2015/summary/.
- ↑ 83.0 83.1 "Ivermectin, 'wonder drug' from Japan: the human use perspective". Proceedings of the Japan Academy. Series B, Physical and Biological Sciences 87 (2): 13–28. 2011. doi:10.2183/pjab.87.13. PMID 21321478. Bibcode: 2011PJAB...87...13C.
- ↑ African Health Leaders: Making Change and Claiming the Future. OUP Oxford. 2014. p. PT158. ISBN 978-0191008412. https://books.google.com/books?id=e0nYBAAAQBAJ&pg=PT158. Retrieved April 6, 2020.
- ↑ "Ivermectin reduces in vivo coronavirus infection in a mouse experimental model". Scientific Reports 11 (1): 7132. March 2021. doi:10.1038/s41598-021-86679-0. PMID 33785846. Bibcode: 2021NatSR..11.7132A.
- ↑ Nelson Textbook of Pediatrics E-Book. Elsevier Health Sciences. 2019. p. 3575. ISBN 978-0323568883. https://books.google.com/books?id=LJuRDwAAQBAJ&pg=PA3867. Retrieved April 6, 2020.
- ↑ Ivermectin for Parasitic Skin Infections of Scabies: A Review of Comparative Clinical Effectiveness, Cost-Effectiveness, and Guidelines. CADTH Rapid Response Reports. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health. 2019. http://www.ncbi.nlm.nih.gov/books/NBK545083/. Retrieved July 4, 2020.
- ↑ Ivermectin for Parasitic Skin Infections of Lice: A Review of Comparative Clinical Effectiveness, Cost-Effectiveness, and Guidelines. CADTH Rapid Response Reports. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health. 2019. http://www.ncbi.nlm.nih.gov/books/NBK545892/. Retrieved July 4, 2020.
- ↑ "Sklice – ivermectin lotion". November 9, 2017. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c74905e6-fc04-4244-880b-98ab0551df21.
- ↑ "Alive Pharmaceutical (P) LTD.: Iver-DT". May 27, 2014. http://alivepharmaceutical.blogspot.com/2014/05/iver-dt.html.
- ↑ "Avermectins, MK-933 and MK-936, for mosquito control". Transactions of the Royal Society of Tropical Medicine and Hygiene 79 (6): 797–799. 1985. doi:10.1016/0035-9203(85)90121-X. PMID 3832491.
- ↑ "Effect of ivermectin on Trypanosoma brucei brucei in experimentally infected mice". Journal of Vector Borne Diseases 49 (3): 143–150. September 2012. PMID 23135008.
- ↑ 93.0 93.1 "Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses". Antiviral Research 126: 117–124. February 2016. doi:10.1016/j.antiviral.2015.12.012. PMID 26752081.
- ↑ "Ivermectin to reduce malaria transmission I. Pharmacokinetic and pharmacodynamic considerations regarding efficacy and safety". Malaria Journal 16 (1): 161. April 2017. doi:10.1186/s12936-017-1801-4. PMID 28434401.
- ↑ "Would ivermectin for malaria control be beneficial in onchocerciasis-endemic regions?". Infectious Diseases of Poverty 8 (1): 77. August 2019. doi:10.1186/s40249-019-0588-7. PMID 31439040.
- ↑ "Repurposing Drugs to Fight Hepatic Malaria Parasites". Molecules 25 (15): 3409. July 2020. doi:10.3390/molecules25153409. PMID 32731386.
- ↑ 97.0 97.1 "Ivermectin treatment in humans for reducing malaria transmission". The Cochrane Database of Systematic Reviews 2021 (6): CD013117. June 2021. doi:10.1002/14651858.CD013117.pub2. PMID 34184757.
- ↑ "Prevention Efforts for Malaria". Current Tropical Medicine Reports 5 (1): 41–50. 2018. doi:10.1007/s40475-018-0133-y. PMID 29629252.
- ↑ "Moxidectin: an oral treatment for human onchocerciasis". Expert Review of Anti-Infective Therapy 18 (11): 1067–1081. November 2020. doi:10.1080/14787210.2020.1792772. PMID 32715787.
- ↑ "Moxidectin for deworming: from trials to implementation". The Lancet. Infectious Diseases 18 (8): 817–819. August 2018. doi:10.1016/S1473-3099(18)30270-6. PMID 29858152.
- ↑ "A new powerful drug to combat river blindness". Lancet 392 (10154): 1170–1172. October 2018. doi:10.1016/S0140-6736(18)30101-6. PMID 29361336.

- ↑ "Ivermectin: enigmatic multifaceted 'wonder' drug continues to surprise and exceed expectations". The Journal of Antibiotics 70 (5): 495–505. May 2017. doi:10.1038/ja.2017.11. PMID 28196978.
- ↑ "A Splendid Gift from the Earth: The Origins and Impact of the Avermectins (Nobel Lecture)". Angewandte Chemie 55 (35): 10190–10209. August 2016. doi:10.1002/anie.201602164. PMID 27435664. https://www.nobelprize.org/prizes/medicine/2015/omura/lecture/. Retrieved April 6, 2020.
- ↑ Andrews' Diseases of the Skin: Clinical Dermatology. Elsevier Health Sciences. 2015. p. 439. ISBN 978-0323319690. https://books.google.com/books?id=Np6cCQAAQBAJ&pg=PA439. Retrieved April 6, 2020. "Ivermectin treatment is emerging as a potential ancillary measure."
- ↑ Treatment of Skin Disease: Comprehensive Therapeutic Strategies. Elsevier Health Sciences. 2017. p. 89. ISBN 978-0702069130. https://books.google.com/books?id=KuM2DwAAQBAJ&pg=PA89. Retrieved April 6, 2020.
- ↑ "Beyond bile acids: targeting Farnesoid X Receptor (FXR) with natural and synthetic ligands". Current Topics in Medicinal Chemistry 14 (19): 2129–2142. 2014. doi:10.2174/1568026614666141112094058. PMID 25388537. https://www.researchgate.net/publication/268231666. Retrieved April 6, 2020.
- ↑ "The antiparasitic drug ivermectin is a novel FXR ligand that regulates metabolism". Nature Communications 4: 1937. 2013. doi:10.1038/ncomms2924. PMID 23728580. Bibcode: 2013NatCo...4.1937J.
- ↑ "Bile Acid Nuclear Receptor Farnesoid X Receptor: Therapeutic Target for Nonalcoholic Fatty Liver Disease". Endocrinology and Metabolism 31 (4): 500–504. December 2016. doi:10.3803/EnM.2016.31.4.500. PMID 28029021.
- ↑ "Ivermectin for preventing and treating COVID-19". The Cochrane Database of Systematic Reviews 2022 (6): CD015017. June 2022. doi:10.1002/14651858.CD015017.pub3. PMID 35726131.
- ↑ "Effect of Early Treatment with Ivermectin among Patients with Covid-19". The New England Journal of Medicine 386 (18): 1721–1731. May 2022. doi:10.1056/NEJMoa2115869. PMID 35353979.
- ↑ "An inconvenient truth: global worming and anthelmintic resistance". Veterinary Parasitology 186 (1–2): 70–78. May 2012. doi:10.1016/j.vetpar.2011.11.048. PMID 22154968.
- ↑ "Anthelmintic resistance to ivermectin and moxidectin in gastrointestinal nematodes of cattle in Europe". International Journal for Parasitology: Drugs and Drug Resistance 5 (3): 163–171. December 2015. doi:10.1016/j.ijpddr.2015.08.001. PMID 26448902.
- ↑ "Efficacy of ivermectin against gastrointestinal nematodes of cattle in Denmark evaluated by different methods for analysis of faecal egg count reduction". International Journal for Parasitology: Drugs and Drug Resistance 6 (3): 241–250. December 2016. doi:10.1016/j.ijpddr.2016.10.004. PMID 27835769.
- ↑ "Ivermectin". Saunders Handbook of Veterinary Drugs (Fourth ed.). W.B. Saunders. January 1, 2016. pp. 420–23. doi:10.1016/B978-0-323-24485-5.00323-5. ISBN 978-0-323-24485-5.
- ↑ "Pharmacogenetics: it's not just about ivermectin in collies". The Canadian Veterinary Journal 47 (12): 1165–1168. December 2006. PMID 17217086.
- ↑ "MDR1 FAQs". Australian Shepherd Health & Genetics Institute, Inc.. http://www.ashgi.org/articles/mdr1.htm.
- ↑ "Multidrug Sensitivity in Dogs". Washington State University's College of Veterinary Medicine. http://vcpl.vetmed.wsu.edu/vcpl-home.
- ↑ "Alberta. Suspected ivermectin toxicity in kittens". The Canadian Veterinary Journal 32 (4): 245. April 1991. PMID 17423775.
- ↑ "Acarexx". Boehringer Ingelheim. April 11, 2016. https://www.bi-vetmedica.com/species/pet/products/acarexx.html.
- ↑ Understanding reptile parasites: from the experts at Advanced Vivarium Systems. Irvine, Calif: Advanced Vivarium Systems. 2007. ISBN 978-1882770908.
- ↑ "Comfortis- spinosad tablet, chewable". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9e5580d1-d8ea-4e8d-9156-1741b13ba8c9.
- ↑ "Comfortis and ivermectin interaction Safety Warning Notification". U.S. Food and Drug Administration (FDA). https://www.fda.gov/animalveterinary/newsevents/cvmupdates/ucm047942.htm.
External links
- "Ivermectin". U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/ivermectin.
- The Carter Center River Blindness (Onchocerciasis) Control Program
- "Ivermectin Topical". https://medlineplus.gov/druginfo/meds/a613011.html.
 |