Chemistry:Alrestatin
From HandWiki
| |
| Names | |
|---|---|
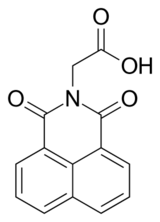
| Preferred IUPAC name
(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H9NO4 | |
| Molar mass | 255.229 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Alrestatin is an inhibitor of aldose reductase, an enzyme involved in the pathogenesis of complications of diabetes mellitus, including diabetic neuropathy.[1][2]
Alrestat was first synthesized in 1969 and was the first aldose reductase inhibitor (ARI) with oral bioavailability to undergo clinical trials, in the late 1970s and early 1980s. Low-quality trials and a high incidence of adverse effects (particularly hepatotoxicity) led to termination of its development, and it was never in clinical use.[3][4] It is structurally related to tolrestat, another ARI that was briefly marketed before being withdrawn in 1997.
Synthesis
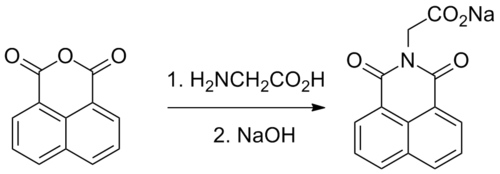
Alrestatin can be synthesized by the reaction of naphthalic anhydride with glycine.[5]
See also
References
- ↑ "Aldose reductase inhibition: studies with alrestatin". Metab Clin Exp 28 (4 Suppl 1): 471–6. April 1979. doi:10.1016/0026-0495(79)90059-3. PMID 122298.
- ↑ "Mechanism of aldose reductase inhibition: binding of NADP+/NADPH and alrestatin-like inhibitors". Biochemistry 33 (23): 7157–65. June 1994. doi:10.1021/bi00189a019. PMID 8003482.
- ↑ Striker, Gary E.; Gueriguian, John L. (1991). Diabetic complications: epidemiology and pathogenetic mechanisms. New York: Raven Press. pp. 293–4. ISBN 0-88167-648-9.
- ↑ Veves, Aristidis (2007). "Aldose reductase inhibitors for the treatment of diabetic neuropathy". Diabetic Neuropathy: Clinical Management. Totowa, NJ: Humana Press. pp. 309–11. ISBN 978-1-59745-311-0. https://books.google.com/books?id=dMKoFySox7kC&pg=PA310. Retrieved 2013-02-13.
- ↑ Ayerst Mckenna & Harrison, U.S. Patent 3,821,383
 |