Chemistry:Di-tert-butyl peroxide
From HandWiki
Short description: DTBP: organic compound consisting of a peroxide group bonded to two tert-butyl groups
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-(tert-Butylperoxy)-2-methylpropane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H18O2 | |
| Molar mass | 146.230 g·mol−1 |
| Density | 0.796 g/cm3 |
| Melting point | −40 °C (−40 °F; 233 K) |
| Boiling point | 109 to 111 °C (228 to 232 °F; 382 to 384 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
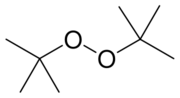
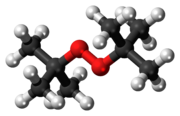
Di-tert-butyl peroxide or DTBP is an organic compound consisting of a peroxide group bonded to two tert-butyl groups. It is one of the most stable organic peroxides, due to the tert-butyl groups being bulky. It is a colorless liquid.[1]
Reactions
The peroxide bond undergoes homolysis at temperatures above 100 °C. For this reason di-tert-butyl peroxide is commonly used as a radical initiator in organic synthesis and polymer chemistry. The decomposition reaction proceeds via the generation of methyl radicals.
DTBP can in principle be used in engines where oxygen is limited, since the molecule supplies both the oxidizer and the fuel.[2]
Toxicity
DTBP is an irritant to the nose, eyes, and skin. It is also flammable, so it should be handled with care.
See also
References
- ↑ RajanBabu, T. V.; Simpkins, Nigel S. (2005). "1,1-Di-tert-butyl Peroxide". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd066.pub2. ISBN 0471936235.
- ↑ Pritchard, H. O.; Clothier, P. Q. E. (1986). "Anaerobic operation of an internal combustion engine". J. Chem. Soc. Chem. Commun. 1986 (20): 1529–1530. doi:10.1039/C39860001529.
External links
- Faraj, Mahmoud K., "Preparation of dialkyl peroxides", US patent 5288919, published 1994-02-22
- Liotta, Frank J., Jr.; Mahmoud K. Faraj & Daniel B. Pourreau et al., "Integrated process for the production of ditertiary butyl peroxide", US patent 5312998, published 1994-05-17
- Pourreau, Daniel B.; Haven S., Jr. Kesling & Frank J., Jr. Liotta et al., "Preparation of dialkyl peroxides", US patent 5371298, published 1994-12-06
 |

