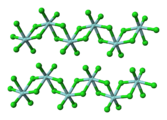
Chemistry:Bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions.[1] The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals.
In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ,[2] with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands.
List of bridging ligands
Virtually all ligands are known to bridge, with the exception of amines and ammonia.[3] Common bridging ligands include most of the common anions.
| Bridging ligand | Name | Example |
|---|---|---|
| OH− |
hydroxide | [Fe 2(OH) 2(H 2O) 8]4+, see olation |
| O2− | oxide | [Cr 2O 7]2−, see polyoxometalate |
| SH− |
hydrosulfido | Cp 2Mo 2(SH) 2S 2 |
| NH− 2 |
amido | HgNH 2Cl |
| N3− | nitride | [Ir 3N(SO 4) 6(H 2O) 3]4−, see metal nitrido complex |
| CO | carbonyl | Fe 2(CO) 9, see bridging carbonyl |
| Cl− |
chloride | Nb 2Cl 10, see halide ligands |
| H− |
hydride | B 2H 6 |
| CN− |
cyanide | approx. Fe 7(CN) 18 (prussian blue), see cyanometalate |
| PPh− 2 |
diphenylphosphide | see transition metal phosphido complexes |
Many simple organic ligands form strong bridges between metal centers. Many common examples include organic derivatives of the above inorganic ligands (R = alkyl, aryl): OR−
, SR−
, NR−
2, NR2− (imido), PR−
2 (phosphido, note the ambiguity with the preceding entry), PR2− (phosphinidino), and many more.
Examples
- Compounds and complexes with bridging ligands
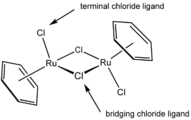
In this ruthenium complex ((benzene)ruthenium dichloride dimer), two chloride ligands are terminal and two are μ2 bridging.
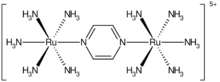
Pyrazine is a bridging ligand in this diruthenium compound, called the Creutz–Taube complex.
In the cobalt cluster Co
3(CO)
9(Ct
Bu), the Ct
Bu ligand is triply bridging, although this aspect is typically not indicated in the formula.In triiron dodecacarbonyl, two CO ligands are bridging and ten are terminal ligands. The terminal and bridging CO ligands interchange rapidly.
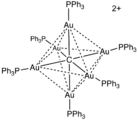
The cluster [Au
6C(PPh
3)
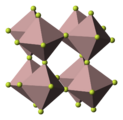
6]2+ features a μ6-carbide ligand, although again, the designator "μ" is not usually used.In rhenium trioxide, the oxide ligands are all μ2. These oxide ligands "glue" together the metal centres.
In the case of ZrCl
4, there are both terminal and doubly bridging chloride ligands.In rhodium(II) acetate, the four acetate groups are bridging ligands.
In VO(HPO
4) · 0.5H2O, pairs of vanadium(IV) centers are bridged by water ligands.[4]
Bonding
For doubly bridging (μ2-) ligands, two limiting representation are 4-electron and 2-electron bonding interactions. These cases are illustrated in main group chemistry by [Me
2Al(μ2−Cl)]
2 and [Me
2Al(μ2−Me)]
2. Complicating this analysis is the possibility of metal–metal bonding. Computational studies suggest that metal-metal bonding is absent in many compounds where the metals are separated by bridging ligands. For example, calculations suggest that Fe
2(CO)
9 lacks an iron–iron bond by virtue of a 3-center 2-electron bond involving one of three bridging CO ligands.[5]
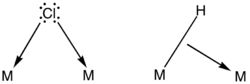
Bridge-terminal exchange
The interchange of bridging and terminal ligands is called bridge-terminal exchange. The process is invoked to explain the fluxional properties of metal carbonyl and metal isocyanide complexes.[6] Some complexes that exhibit this process are cobalt carbonyl and cyclopentadienyliron dicarbonyl dimer:
- Co2(μ-CO)2(CO)6 ⇌ Co2(μ-CO)2(CO)4(CO)2
- (C5H5)2Fe2(μ-CO)2(CO)2 ⇌ (C5H5)2Fe2(μ-CO)2(CO)2
These dynamic processes, which are degenerate, proceed via an intermediate where the CO ligands are all terminal, i.e. (CO)
4Co–Co(CO)
4 and (C
5H
5)(CO)
2Fe–Fe(CO)
2C
5H
5.
Polyfunctional ligands
Polyfunctional ligands can attach to metals in many ways and thus can bridge metals in diverse ways, including sharing of one atom or using several atoms. Examples of such polyatomic ligands are the oxoanions CO2−
3 and the related carboxylates, PO3−
4, and the polyoxometalates. Several organophosphorus ligands have been developed that bridge pairs of metals, a well-known example being Ph
2PCH
2PPh
2.
See also
- Bridging carbonyl
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "bridging ligand". doi:10.1351/goldbook.B00741
- ↑ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN:0-85404-438-8. pp. 163–165. Electronic version.
- ↑ Werner, H. (2004). "The Way into the Bridge: A New Bonding Mode of Tertiary Phosphanes, Arsanes, and Stibanes". Angew. Chem. Int. Ed. 43 (8): 938–954. doi:10.1002/anie.200300627. PMID 14966876.
- ↑ Koo, H.-J.; Whangbo, M.; VerNooy, P. D.; Torardi, C. C.; Marshall, W. J. (2002). "Flux growth of vanadyl pyrophosphate, (VO)2P2O7, and spin dimer analysis of the spin exchange interactions of (VO)2P2O7 and vanadyl hydrogen phosphate, VO(HPO4).0.5H2O.". Inorg. Chem. 41 (18): 4664–72. doi:10.1021/ic020249c. PMID 12206689.
- ↑ 5.0 5.1 Green, J. C.; Green, M. L. H.; Parkin, G. (2012). "The occurrence and representation of three-centre two-electron bonds in covalent inorganic compounds". Chem. Commun. 2012 (94): 11481–503. doi:10.1039/c2cc35304k. PMID 23047247.
- ↑ Adams, R. D.; Cotton, F. A. (1973). "Pathway of Bridge-Terminal Ligand Exchange in Some Binuclear Metal Carbonyls. Bis(pentahapto-cyclopentadienyldicarbonyliron) and Its Di(methyl Isocyanide) Derivative and Bis(pentahapto-cyclopentadienylcarbonylnitrosylmanganese)". Journal of the American Chemical Society 95 (20): 6589–6594. doi:10.1021/ja00801a012.
 |