Chemistry:Total synthesis of morphine and related alkaloids
Synthesis of morphine-like alkaloids in chemistry describes the total synthesis of the natural morphinan class of alkaloids that includes codeine, morphine, oripavine, and thebaine and the closely related semisynthetic analogs methorphan, buprenorphine, hydromorphone, hydrocodone, isocodeine, naltrexone, nalbuphine, oxymorphone, oxycodone, and naloxone.[1][2]
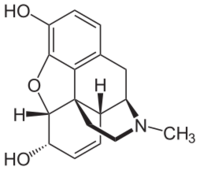
The structure of morphine is not particularly complex, however the electrostatic polarization of adjacent bonded atoms does not alternate uniformly throughout the structure. This "dissonant connectivity" makes bond formation more difficult and therefore significantly complicates any synthetic strategy that is applied to this family of molecules.[2]
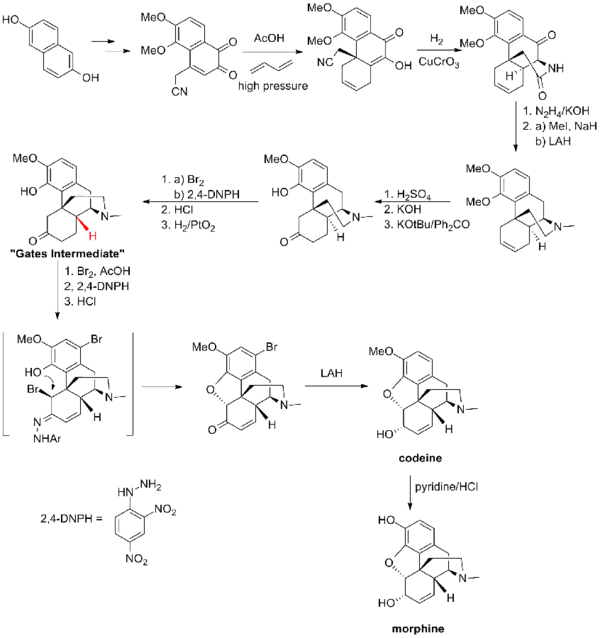
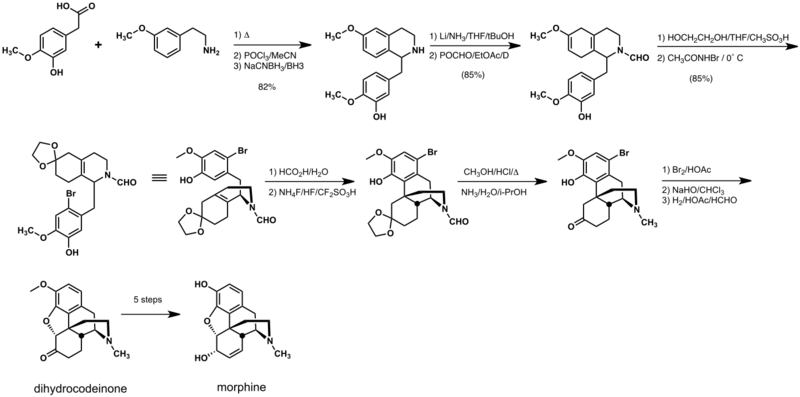
The first morphine total synthesis, devised by Marshall D. Gates, Jr. in 1952 remains a widely used example of total synthesis.[3] This synthesis took a total of 31 steps and proceeded in 0.06% overall yield. The hydrocodone synthesis of Kenner C. Rice is one of the most efficient and proceeds in 30% overall yield in 14 steps.[4] At 9 steps, the Barriault route is the shortest to date, but contains a number of low-yielding steps and is racemic.[5]
Several other syntheses were reported, notably by the research groups of Evans,[6] Fuchs,[7] Parker,[8] Overman,[9] Mulzer-Trauner,[10] White,[11] Taber,[12] Trost,[13] Fukuyama,[14] Guillou,[15] Stork,[16] Magnus,[17] Smith,[18] and Barriault.[5]
Gates synthesis
Gates' total synthesis of morphine[3] provided a proof of the structure of morphine proposed by Robinson in 1925.[19] This synthesis of morphine features one of the first examples of the Diels-Alder reaction in the context of total synthesis.[3]
Rice synthesis
The Rice synthesis follows a biomimetic route and is the most efficient reported to date. A key step is the Grewe cyclization that is analogous to the cyclization of reticuline that occurs in morphine biosynthesis.[4]
References
- ↑ "Recent advances in the synthesis of morphine and related alkaloids". Chemistry of Opioids. 299. 2011. 1–28. doi:10.1007/128_2010_73. ISBN 978-3-642-18106-1.
- ↑ 2.0 2.1 "Synthesis of morphine alkaloids and derivatives". Alkaloid Synthesis. 309. 2012. 33–66. doi:10.1007/128_2011_133. ISBN 978-3-642-25528-1. "Morphine's synthesis remains a serious challenge to this day."
- ↑ 3.0 3.1 3.2 "The Synthesis of Morphine". Journal of the American Chemical Society 78 (7): 1380–1393. April 1956. doi:10.1021/ja01588a033.
- ↑ 4.0 4.1 Rice KC (July 1980). "Synthetic opium alkaloids and derivatives. A short total synthesis of (+/-)-dihydrothebainone, (+/-)-dihydrocodeinone, and (+/-)-nordihydrocodeinone as an approach to a practical synthesis of morphine, codeine, and congeners". The Journal of Organic Chemistry 45 (15): 3135–3137. doi:10.1021/jo01303a045.
- ↑ 5.0 5.1 Brousseau, Julie; Xolin, Amandine; Barriault, Louis (2019). "A Nine-Step Formal Synthesis of (±)-Morphine" (in en). Organic Letters 21 (5): 1347–1349. doi:10.1021/acs.orglett.9b00044. PMID 30785291. https://figshare.com/articles/A_Nine-Step_Formal_Synthesis_of_-Morphine/7745963.
- ↑ "Studies directed towards the total synthesis of morphine alkaloids". Tetrahedron Letters 23 (3): 285–288. January 1982. doi:10.1016/S0040-4039(00)86810-0.
- ↑ "Studies culminating in the total synthesis of (dl)-morphine". The Journal of Organic Chemistry 53 (20): 4694–4708. September 1988. doi:10.1021/jo00255a008.
- ↑ "Convergent synthesis of (+/-)-dihydroisocodeine in 11 steps by the tandem radical cyclization strategy. A formal total synthesis of (+/-)-morphine". Journal of the American Chemical Society 114 (24): 9688–9689. November 1992. doi:10.1021/ja00050a075.
- ↑ "Asymmetric synthesis of either enantiomer of opium alkaloids and morphinans. Total synthesis of (−)- and (+)-dihydrocodeinone and (−)- and (+)-morphine". Journal of the American Chemical Society 115 (23): 11028–11029. November 1993. doi:10.1021/ja00076a086.
- ↑ "Formal Total Synthesis of(—)-Morphine by Cuprate Conjugate Addition". Angewandte Chemie International Edition in English 35 (2324): 2830–2832. December 1996. doi:10.1002/anie.199628301.
- ↑ "Asymmetric Total Synthesis of (+)-Codeine via Intramolecular Carbenoid Insertion". The Journal of Organic Chemistry 64 (21): 7871–7884. October 1999. doi:10.1021/jo990905z.
- ↑ "Synthesis of (-)-morphine". Journal of the American Chemical Society 124 (42): 12416–7. October 2002. doi:10.1021/ja027882h. PMID 12381175.
- ↑ "Enantioselective synthesis of (-)-codeine and (-)-morphine". Journal of the American Chemical Society 124 (49): 14542–3. December 2002. doi:10.1021/ja0283394. PMID 12465957. https://figshare.com/articles/Enantioselective_Synthesis_of_-Codeine_and_-Morphine/3646293.
- ↑ "Total synthesis of (+/-)-morphine". Organic Letters 8 (23): 5311–3. November 2006. doi:10.1021/ol062112m. PMID 17078705.
- ↑ "Diastereoselective total synthesis of (+/-)-codeine". Chemistry: A European Journal 14 (22): 6606–8. 2008. doi:10.1002/chem.200800744. PMID 18561354.
- ↑ "Regiospecific and stereoselective syntheses of (+/-) morphine, codeine, and thebaine via a highly stereocontrolled intramolecular 4 + 2 cycloaddition leading to a phenanthrofuran system". Journal of the American Chemical Society 131 (32): 11402–6. August 2009. doi:10.1021/ja9038505. PMID 19624126. https://figshare.com/articles/Regiospecific_and_Stereoselective_Syntheses_of_Morphine_Codeine_and_Thebaine_via_a_Highly_Stereocontrolled_Intramolecular_4_2_Cycloaddition_Leading_to_a_Phenanthrofuran_System/2834536.
- ↑ "Concise syntheses of (-)-galanthamine and (+/-)-codeine via intramolecular alkylation of a phenol derivative". Journal of the American Chemical Society 131 (44): 16045–7. November 2009. doi:10.1021/ja9085534. PMID 19835379. https://figshare.com/articles/Concise_Syntheses_of_Galanthamine_and_Codeine_via_Intramolecular_Alkylation_of_a_Phenol_Derivative/2813797.
- ↑ "A Cascade Strategy Enables a Total Synthesis of (±)-Morphine". Angewandte Chemie 55 (46): 14306–14309. November 2016. doi:10.1002/anie.201608526. PMID 27735107.
- ↑ "Constitution of codeine and thebaine". Memoirs of the Literary and Philosophical Society of Manchester 69: 79–86. 1925.
External links
- Morphine Total Syntheses @ SynArchive.com
- Wilson T (2006). "Synthesis of Morphine Alkaloids". Professor Scott E. Denmark Research Group Presentations. University of Illinois at Urbana-Champaign. http://www.scs.illinois.edu/denmark/presentations/2006/gm-2006-01n31.pdf.
 |