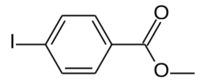
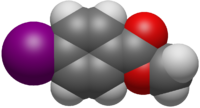
Chemistry:Methyl 4-iodobenzoate
From HandWiki
| |
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl 4-iodobenzoate | |
| Other names
Methyl p-iodobenzoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C8H7IO2 | |
| Molar mass | 262.046 g·mol−1 |
| Appearance | white solid |
| Melting point | 114 °C (237 °F; 387 K)[1] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H315, H319, H335, H411 | |
| P261, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P271, P273, P280, P302+352, P304+340, P305+351+338, P319Script error: No such module "Preview warning".Category:GHS errors, P321, P332+317Script error: No such module "Preview warning".Category:GHS errors, P337+317Script error: No such module "Preview warning".Category:GHS errors, P362+364Script error: No such module "Preview warning".Category:GHS errors, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Methyl 4-iodobenzoate, or methyl p-iodobenzoate, is an organic compound with the formula IC6H4COOCH3.[3] It is the methyl ester of 4-iodobenzoic acid, or may also be viewed as an iodinated derivative of methyl benzoate.
Preparation
Methyl 4-iodobenzoate may be prepared by the Fischer esterification of 4-iodobenzoic acid with methanol.[4]
Reactions
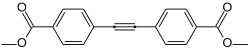
The aryl-iodide functionality of methyl 4-iodobenzoate may undergo coupling reactions, such as a symmetrical Sonogashira coupling with trimethylsilylacetylene (with the TMSA deprotected to acetylene in situ) to form dimethyl 4,4'-(ethyne-1,2-diyl)dibenzoate.[4][5]
References
- ↑ "Methyl 4-iodobenzoate". https://www.chemspider.com/Chemical-Structure.62484.html.
- ↑ "Methyl 4-iodobenzoate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/69273#section=Safety-and-Hazards.
- ↑ PubChem. "Methyl 4-iodobenzoate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/Methyl-4-iodobenzoate.
- ↑ 4.0 4.1 Gadzikwa, Tendai; Zeng, Bi-Shun; Hupp, Joseph T.; Nguyen, SonBinh T. (2008). "Ligand-elaboration as a strategy for engendering structural diversity in porous metal–organic framework compounds". Chemical Communications (31): 3672–3674. doi:10.1039/B714160B. PMID 18665295.
- ↑ Mio, Matthew J.; Kopel, Lucas C.; Braun, Julia B.; Gadzikwa, Tendai L.; Hull, Kami L.; Brisbois, Ronald G.; Markworth, Christopher J.; Grieco, Paul A. (2002). "One-Pot Synthesis of Symmetrical and Unsymmetrical Bisarylethynes by a Modification of the Sonogashira Coupling Reaction". Organic Letters 4 (19): 3199–3202. doi:10.1021/ol026266n. PMID 12227748.
 |