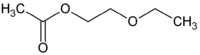
Chemistry:2-Ethoxyethyl acetate
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Ethoxyethyl acetate | |
| Other names
Ethyglycol acetate; Ethylene glycol mono ethyl ether acetate; 2-EEA; Ethoxyethanol acetate; EGA; Cellosolve acetate; Ethoxol acetate; Oxidol acetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1172 |
| |
| |
| Properties | |
| C6H12O3 | |
| Molar mass | 132.159 g·mol−1 |
| Density | 0.973 (20 °C) |
| Melting point | < -62 °C |
| Boiling point | 156 °C (313 °F; 429 K) |
| 229 g/L (20 °C) | |
| Vapor pressure | 270 Pa (20 °C) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H226, H302, H312, H332, H360 | |
| P201, P280, P308, P313 | |
| Flash point | 51 °C (124 °F; 324 K) |
| 380 °C (716 °F; 653 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Ethoxyethyl acetate is an organic compound with the formula CH3CH2OCH2CH2O2CCH3. It is the ester of ethoxyethanol and acetic acid. A colorless liquid, it is partially soluble in water.[1]
Properties
2-Ethoxyethyl acetate is a liquid at room temperature that is used as a solvent. It can be absorbed through inhalation, ingestion, and dermally and should be avoided. It may form an explosive mixture with air. It is also incompatible with strong acids, strong alkalis and nitrates. It may form unstable peroxides and it can soften many plastics, attack plastics, rubber and coatings.[2][3]
Uses
2-Ethoxyethyl acetate has been used to dissolve polyester and short oil alkyd resins.[4] It has also been used in coatings, dyes, insecticides, soaps and cosmetics.[5] It is also a solvent for nitro-cellulose and is being used for the same applications as ethyl glycol[4][6]
In automobile lacquers it has been used to reduce evaporation and to impart a high gloss.[7]
Metabolism
2-Ethoxyethyl acetate is rapidly metabolized to 2-ethoxyethanol in the blood via hydrolysis. Then, 2-ethoxyethanol is metabolized, mainly by alcohol dehydrogenase, to 2-ethoxyacetaldehyde, which is further metabolized by aldehyde dehydrogenase to 2-ethoxyacetic acid (2-EAA) in the liver. These two compounds are the likely active metabolites, which are thought to be involved in some of the toxic effects. Also in the liver, ethylene glycol is produced.[8][9][10] All this reactions belong to the phase I of the biotransformation process. In rats, EAA can be conjugated with glycine or may suffer O-deethylated. After this, it can be metabolized to carbon dioxide. An extra pathway in these animals involves microsomal P450 mixed function oxidases, with deethylation producing acetaldehyde and ethylene glycol.[9]
Safety
2-Ethoxyethyl acetate can cause a slight skin and eye irritation after exposure.[6] High exposure can lead to kidney damage and paralysis.[11] In animals, reproductive and teratogenic effects have been observed.[11]
References
- ↑ Ketting, Joris. "Ethyl Glycol Acetate - KH Chemicals". KH Chemicals. http://www.khchemicals.com/product-categories/acetates/ethylglycolacetate/.
- ↑ Pohanish, Richard P. (2017-06-05). Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens. William Andrew. ISBN 978-0-323-38969-3. https://books.google.com/books?id=PlDpCAAAQBAJ&dq=2-ethoxyethyl+acetate&pg=PA1261.
- ↑ 2-Ethoxyethyl acetate: Summary Risk Assessment (Report). European Chemicals Agency. 2008. https://echa.europa.eu/documents/10162/d02e2d1c-0f53-4cf6-aec8-f1697fcf2db3.
- ↑ 4.0 4.1 JETLAG. "Ethyl glycol acetate - Coating Terms - Kansai Altan" (in tr). http://www.kansaialtan.com/en/terms/ara/Ethyl%20glycol%20acetate.
- ↑ "Pulmonary and percutaneous absorption of 2-propoxyethyl acetate and 2-ethoxyethyl acetate in beagle dogs". Environmental Health Perspectives 57: 177–83. August 1984. doi:10.2307/3429914. PMID 6499802.
- ↑ 6.0 6.1 Proctor, Nick H.; Hughes, James P.; Hathaway, Gloria J. (2004). Proctor and Hughes' Chemical Hazards of the Workplace. John Wiley & Sons. ISBN 978-0-471-26883-3. https://books.google.com/books?id=y3-Ef3y53PkC&dq=2-ethoxyethyl+acetate&pg=PR1.
- ↑ Montgomery, John H. (2010-12-12). Groundwater Chemicals Desk Reference, 3rd Edition. CRC Press. ISBN 9781420032765. https://books.google.com/books?id=bc8sXbYv7qMC&dq=2-ethoxyethyl+acetate&pg=PA1229.
- ↑ "Chemical Sampling Information | 2-Ethoxyethyl acetate". https://www.osha.gov/dts/chemicalsampling/data/CH_239300.html.
- ↑ 9.0 9.1 "Selected Alkoxyethanols: 2-Ethoxyethanol and 2-Eropoxyethanol". Concise International Chemical Assessment Document 67. World Health Organization. 2009. https://www.who.int/ipcs/publications/cicad/ethoxy_propoxyethanol.pdf.
- ↑ "A toxicokinetic study of inhaled ethylene glycol ethyl ether acetate and validation of a physiologically based pharmacokinetic model for rat and human". Toxicology and Applied Pharmacology 165 (1): 63–73. May 2000. doi:10.1006/taap.2000.8927. PMID 10814554.
- ↑ 11.0 11.1 "2-Ethoxyethyl acetate". NIOSH Pocket Guide to Chemical Hazards. Centers for Disease Control and Prevention. https://www.cdc.gov/niosh/npg/npgd0259.html.
 |

