Chemistry:Schiff base
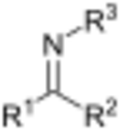
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure R1
R2
C=NR3
(R3
= alkyl or aryl, but not hydrogen).[1][2] They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimines depending on their structure. Anil refers to a common subset of Schiff bases: imines derived from anilines.[3] The term can be synonymous with azomethine which refers specifically to secondary aldimines (i.e. R–CH=NR' where R' ≠ H).[4]
Synthesis
Schiff bases can be synthesized from an aliphatic or aromatic amine and a carbonyl compound by nucleophilic addition forming a hemiaminal, followed by a dehydration to generate an imine. In a typical reaction, 4,4'-oxydianiline reacts with o-vanillin:[5]
Biochemistry
Schiff bases have been investigated in relation to a wide range of contexts, including antimicrobial, antiviral and anticancer activity. They have also been considered for the inhibition of amyloid-β aggregation.[6]
Schiff bases are common enzymatic intermediates where an amine, such as the terminal group of a lysine residue, reversibly reacts with an aldehyde or ketone of a cofactor or substrate. The common enzyme cofactor pyridoxal phosphate (PLP) forms a Schiff base with a lysine residue and is transaldiminated to the substrate(s).[7] Similarly, the cofactor retinal forms a Schiff base in rhodopsins, including human rhodopsin (via Lysine 296), which is key in the photoreception mechanism.
Coordination chemistry
The term Schiff base is normally applied to these compounds when they are being used as ligands to form coordination complexes with metal ions. One example is Jacobsen's catalyst. The imine nitrogen is basic and exhibits pi-acceptor properties. Several, especially the diiminopyridines are noninnocent ligands. Many Schiff base ligands are derived from alkyl diamines and aromatic aldehydes.[8]
Copper(II) complex of the Schiff base ligand salicylaldoxime.
Salen is a common tetradentate ligand that becomes deprotonated upon complexation.
Jacobsen's catalyst is derived from a chiral salen ligand.
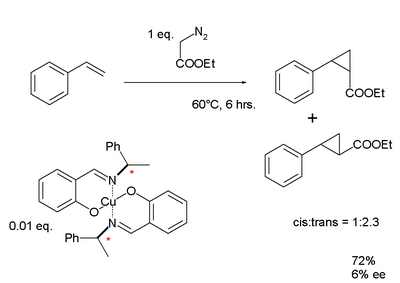
Chiral Schiff bases were one of the first ligands used for asymmetric catalysis. In 1968 Ryōji Noyori developed a copper-Schiff base complex for the metal-carbenoid cyclopropanation of styrene.[9] Schiff bases have also been incorporated into metal–organic frameworks (MOF).[10]
Conjugated Schiff bases
Conjugated Schiff bases absorb strongly in the UV-visible region of the electromagnetic spectrum. This absorption is the basis of the anisidine value, which is a measure of oxidative spoilage for fats and oils.
Historic references
- Schiff, Hugo (1864). "Mittheilungen aus dem Universitäts-laboratorium in Pisa: 2. Eine neue Reihe organischer Basen" (in de). Annalen der Chemie und Pharmacie 131: 118–119. doi:10.1002/jlac.18641310113. https://babel.hathitrust.org/cgi/pt?id=uva.x002457965;view=1up;seq=130.
- Schiff, Ugo (1866). "Sopra una nova serie di basi organiche" (in it). Giornale di Scienze Naturali ed Economiche 2: 201–257. http://babel.hathitrust.org/cgi/pt?id=hvd.32044106232283;view=1up;seq=207.
- Schiff, Hugo (1866). "Eine neue Reihe organischer Diamine" (in de). Annalen der Chemie und Pharmacie, Supplementband 3: 343–370. https://babel.hathitrust.org/cgi/pt?id=uc1.b3483657;view=1up;seq=353.
- Schiff, Hugo (1866). "Eine neue Reihe organischer Diamine. Zweite Abtheilung." (in de). Annalen der Chemie und Pharmacie 140: 92–137. doi:10.1002/jlac.18661400106. https://babel.hathitrust.org/cgi/pt?id=uva.x002457971;view=1up;seq=488.
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Schiff base". doi:10.1351/goldbook.S05498
- ↑ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 1281, ISBN 978-0-471-72091-1, https://books.google.com/books?id=JDR-nZpojeEC&printsec=frontcover
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "anil". doi:10.1351/goldbook.A00357
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "azomethines". doi:10.1351/goldbook.A00564
- ↑ Jarrahpour, A. A.; M. Zarei (February 24, 2004). "Synthesis of 2-({[4-(4-{[(E)-1-(2-hydroxy-3-methoxyphenyl)methylidene amino}phenoxy)phenyl imino}methyl)- 6 -methoxy phenol". Molbank M352. ISSN 1422-8599. http://www.mdpi.net/molbank/molbank2004/m0352.htm. Retrieved February 22, 2010.
- ↑ Bajema, Elizabeth A.; Roberts, Kaleigh F.; Meade, Thomas J. (2019). "Chapter 11. Cobalt-Schiff Base Complexes:Preclinical Research and Potential Therapeutic Uses". in Sigel, Astrid; Freisinger, Eva; Sigel, Roland K. O. et al.. Essential Metals in Medicine:Therapeutic Use and Toxicity of Metal Ions in the Clinic. Metal Ions in Life Sciences. 19. Berlin: de Gruyter GmbH. pp. 267–301. doi:10.1515/9783110527872-017. ISBN 978-3-11-052691-2.
- ↑ Eliot, A. C.; Kirsch, J. F. (2004). "PYRIDOXALPHOSPHATEENZYMES: Mechanistic, Structural, and Evolutionary Considerations". Annual Review of Biochemistry 73: 383–415. doi:10.1146/annurev.biochem.73.011303.074021. PMID 15189147.
- ↑ Hernández-Molina, R.; Mederos, A. (2003). "Acyclic and Macrocyclic Schiff Base Ligands". Comprehensive Coordination Chemistry II. pp. 411–446. doi:10.1016/B0-08-043748-6/01070-7. ISBN 9780080437484.
- ↑ Nozaki, H.; Takaya, H.; Moriuti, S.; Noyori, R. (1968). "Homogeneous catalysis in the decomposition of diazo compounds by copper chelates: Asymmetric carbenoid reactions". Tetrahedron 24 (9): 3655–3669. doi:10.1016/S0040-4020(01)91998-2.
- ↑ Uribe-Romo, Fernando J.; Hunt, Joseph R.; Furukawa, Hiroyasu; KlöCk, Cornelius; o'Keeffe, Michael; Yaghi, Omar M. (2009). "A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework". J. Am. Chem. Soc. 131 (13): 4570–4571. doi:10.1021/ja8096256. PMID 19281246.
 |