Chemistry:Cobalt germanide
From HandWiki
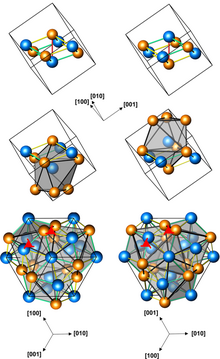 Structures of left-handed and right-handed cubic CoGe crystals (3 presentations, with different numbers of atoms per unit cell; orange atoms are Ge)
| |
| Names | |
|---|---|
| IUPAC name
Cobalt germanide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CoGe | |
| Molar mass | 131.56 g/mol |
| 1.3×10−6 emu/g[1] | |
| Structure | |
| Monoclinic[1] | |
| C2/m (No. 12), mS16 | |
a = 1.165 nm, b = 0.3807 nm, c = 0.4945 nm α = 90°, β = 101.1°, γ = 90°
| |
Formula units (Z)
|
8 |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Cobalt silicide |
Other cations
|
Iron germanide Manganese germanide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cobalt germanide (CoGe) is an intermetallic compound, a germanide of cobalt.
Cubic CoGe crystals (space group P213, cP8, a = 0.4631 nm) can be produced by processing a mixture of Co and Ge powders at a pressure of 4 GPa and a temperature of 800–1000 °C for 1 to 3 hours. They have no inversion center, and are therefore helical, with right-hand and left-handed chiralities. The cubic CoGe is metastable, and converts into a monoclinic phase upon subsequent heating to 600 °C at ambient pressure.[1]
Cubic CoGe is an antiferromagnet with a transition temperature Tc of 132 K.[1]
References
- ↑ 1.0 1.1 1.2 1.3 Takizawa, H.; Sato, T.; Endo, T.; Shimada, M. (1988). "High-pressure synthesis and electrical and magnetic properties of MnGe and CoGe with the cubic B20 structure". Journal of Solid State Chemistry 73 (1): 40–46. doi:10.1016/0022-4596(88)90051-5. Bibcode: 1988JSSCh..73...40T.
 |

