Chemistry:Cobalt(III) nitrate
| |
| Names | |
|---|---|
| IUPAC name
Cobalt(III) nitrate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| Co(NO3)3 | |
| Molar mass | 244.96 g/mol |
| Appearance | green crystals hygroscopic |
| Density | 2.49 g/cm3 |
| 5.07 g/100 mL | |
| Structure | |
| cubic | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cobalt(III) nitrate is an inorganic compound with the chemical formula Co(NO3)3.[1] It is a green, diamagnetic solid that sublimes at ambient temperature.[2]
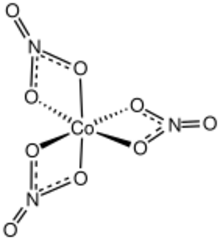
Structure
The compound is a molecular coordination complex. The three bidentate nitrate ligands give a distorted octahedral arrangement.[3] The nitrate ligands are planar. With D3 symmetry, the molecule is chiral. The Co-O bond lengths are about 190 pm long. The O-Co-O angles for the two oxygens in the same nitrate is about 68 degrees.[4] The same geometry seems to persist in carbon tetrachloride solution.[3]
Preparation and reactions
Cobalt(III) nitrate can be prepared by the reaction of dinitrogen pentoxide N2O5 with cobalt(III) fluoride CoF3.[3] It can be purified by vacuum sublimation at 40 °C.[4][2]
Cobalt(III) nitrate oxidizes water, the initial green solution rapidly turns pink, with formation of cobalt(II) ions and release of oxygen.[1] Cobalt(III) nitrate can be intercalated in graphite, in the ratio of 1 molecule for each 12 carbon atoms.[2]
See also
- Cobalt(III) fluoride
- Cobalt(III) chloride
- Cobalt(III) hydroxide
- Iron(III) nitrate
- Iron(III) oxalate
References
- ↑ 1.0 1.1 W. Levason and C. A. McAuliffe (1974): "Higher oxidation state chemistry of iron, cobalt, and nickel". Coordination Chemistry Reviews, volume 12, issue 2, pages 151-184. doi:10.1016/S0010-8545(00)82026-3
- ↑ 2.0 2.1 2.2 E. Stumpp, G. Nietfeld, K. Steinwede, and K. D. Wageringel (1983) "Reaction of anhydrous metal nitrates with graphite". Synthetic Metals, Evolume 7, issues 1–2, pages 143-151. doi:10.1016/0379-6779(83)90097-8
- ↑ 3.0 3.1 3.2 R. J. Fereday, N. Logan and D. Sutton (1969): "Anhydrous cobalt(III) nitrate: preparation, spectra, and reactions with some organic ligands". Journal of the Chemical Society A: Inorganic, Physical, Theoretical, volume 1969, issue 0, pages 2699-2703. doi:10.1039/J19690002699
- ↑ 4.0 4.1 J. Hilton and S. C. Wallwork (1968): "The crystal structure of cobalt(III) nitrate", Chemical Communications, volume 1968, issue 15, pages 871-871. doi:10.1039/C19680000871
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO3)−4 | C | NO−3, NH4NO3 |
O | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3, Fe(NO3)2 |
Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3, TlNO3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd(NO3)3 | Pm | Sm | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac(NO3)3 | Th(NO3)4 | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |

