Chemistry:Cobalt(II) oxalate
From HandWiki

| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| CoC2O4 | |
| Molar mass | 146.9522 g/mol |
| Appearance | gray/pink powder |
| Odor | odorless |
| Density | 3.01 g/cm3 |
| Melting point | 250 °C (482 °F; 523 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
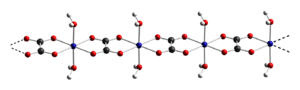
Cobalt(II) oxalate is the inorganic compound with the formula of CoC2O4. Like other simple inorganic oxalates, it is a coordination polymer. The oxalate ligands bridge of Co(OH2)2 centres. Each cobalt adopts octahedral coordination geometry.[1]
It is used in the preparation of cobalt catalysts, and cobalt metal powder for powder-metallurgical applications. It is made in process of recycling lithium-ion batteries, where the cobalt is obtained from cathode material (LiCoO2) by leaching with sulfuric acid and then precipitated with ammonium oxalate.[citation needed]
Related compounds
Many cobalt(III) oxalate complexes are known, including [Co(C2O4)3]3- and [Co(C2H4(NH2)2)C2O4)2]−.[2] [3]
References
- ↑ Bacsa, J.; Eve, D.; Dunbar, K. R. (2005). "catena-Poly[[diaquacobalt(II)]-μ-oxalato]". Acta Crystallogr. C 61 (Pt 1): m58–m60. doi:10.1107/S0108270104030409. PMID 15640580.
- ↑ Kauffman, George B.; Takahashi, Lloyd T.; Sugisaka, Nobuyuki (1966). "Resolution of the Trioxalatocobaltate(III) Ion". Inorganic Syntheses 8: 207–211. doi:10.1002/9780470132395.ch55.
- ↑ Worrell, J. H.; Kipp, E. B. (1972). "Resolution of the (Ethylenediamine)bis(oxalato)cobaltate(III) Ion". Inorganic Syntheses 13: 195–202. doi:10.1002/9780470132449.ch40.
 |

