Chemistry:Iron germanide
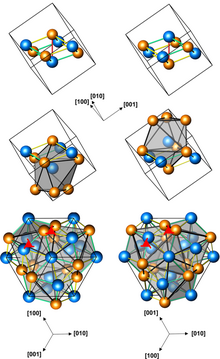 Structures of left-handed and right-handed FeGe crystals (3 presentations, with different numbers of atoms per unit cell; orange atoms are Ge)
| |
| Names | |
|---|---|
| IUPAC name
Iron germanide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| FeGe | |
| Molar mass | 128.47 g/mol |
| Structure | |
| Cubic[1] | |
| P213 (No. 198), cP8 | |
a = 0.4689 nm
| |
Formula units (Z)
|
4 |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Iron silicide |
Other cations
|
Manganese germanide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Iron germanide (FeGe) is an intermetallic compound, a germanide of iron. At ambient conditions it crystallizes in three polymorphs with monoclinic, hexagonal and cubic structures. The cubic polymorph has no inversion center, it is therefore helical, with right-hand and left-handed chiralities.[1]
Magnetism
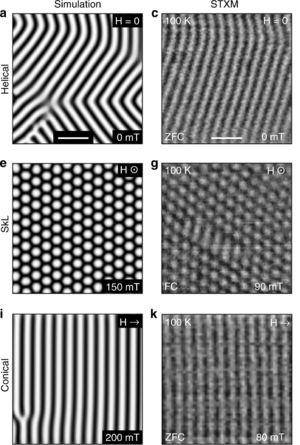
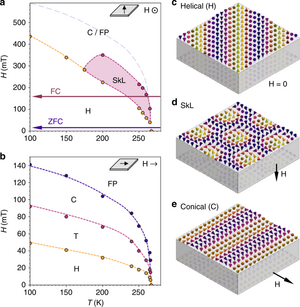
FeGe is extensively studied for its unusual magnetic properties. Electron spins in this material show dissimilar, yet regular spatial arrangements at different values of applied magnetic field. Those arrangements are named helical, skyrmion lattice, and conical. They can be controlled not only by temperature and magnetic field, but also by electric current, and the current density required for manipulating skyrmions (~106 A/m2) is approximately one million times smaller than that needed for moving magnetic domains in traditional ferromagnets. As a result, skyrmions have potential application in ultrahigh-density magnetic storage devices.[2]
The helical, conical and skyrmion structures are not unique to FeGe; they are also found in MnSi, MnGe and similar compounds, but contrary to those materials, the observation of magnetic ordering patterns in FeGe does not require cryogenic cooling.[2] The disadvantage of FeGe over MnSi is its polymorphism, which hinders the growth of large homogeneous crystals.[1]
Synthesis
Polycrystalline FeGe
Polycrystalline FeGe is produced by vacuum arc remelting, spark plasma sintering, or high-pressure high-temperature treatment of a mixture of elemental iron and germanium. Single crystals of FeGe ca. 1 mm in size can be grown from the powder using a chemical transport reaction and iodine as transporting agent. The source temperature is maintained at 450 °C and the temperature gradient at ca. 50 °C across the reaction tube, over 1–2 weeks.[3][4]
FeGe thin film
FeGe films can be epitaxially grown on Si (111) using MBE. The thin film FeGe is polycrystalline with ± 30° in-plane rotations around [111] out-of-plane axis.[5] Theoretical simulations indicate that FeGe thin film can hold skyrmion cylinder or chiral bobber phases, which were recently imaged in a 35 nm plan-view FeGe thin film using Lorentz STEM/TEM.[5]
Structure
Iron germanide is a non-stoichiometric compound where the Ge:Fe ratio often deviates from 1. The Fe2Ge3 compound is a Nowotny phase exhibiting a chimney ladder structure. It is a semiconductor with a band gap or 0.03 eV.[6]
References
- ↑ Jump up to: 1.0 1.1 1.2 Lebech, B; Bernhard, J; Freltoft, T (1989). "Magnetic structures of cubic FeGe studied by small-angle neutron scattering". Journal of Physics: Condensed Matter 1 (35): 6105–6122. doi:10.1088/0953-8984/1/35/010. Bibcode: 1989JPCM....1.6105L.
- ↑ Jump up to: 2.0 2.1 Nagaosa, Naoto; Tokura, Yoshinori (2013). "Topological properties and dynamics of magnetic skyrmions". Nature Nanotechnology 8 (12): 899–911. doi:10.1038/nnano.2013.243. PMID 24302027. Bibcode: 2013NatNa...8..899N.
- ↑ Hiroto, Takanobu; So, Yeong-Gi; Kimura, Kaoru (2018). "Synthesis and Thermal Stability of B20-Type TMGe (TM = Mn, Fe and Co) Intermetallic Compounds Prepared by Mechanical Milling". Materials Transactions 59 (6): 1005–1008. doi:10.2320/matertrans.M2018016.
- ↑ Birch, M. T.; Cortés-Ortuño, D.; Turnbull, L. A.; Wilson, M. N.; Groß, F.; Träger, N.; Laurenson, A.; Bukin, N. et al. (2020). "Real-space imaging of confined magnetic skyrmion tubes". Nature Communications 11 (1): 1726. doi:10.1038/s41467-020-15474-8. PMID 32265449. Bibcode: 2020NatCo..11.1726B.
- ↑ Jump up to: 5.0 5.1 Wang, Binbin; Bagués, Núria; Liu, Tao; Kawakami, Roland K.; McComb, David W. (2022-01-01). "Extracting weak magnetic contrast from complex background contrast in plan-view FeGe thin films" (in en). Ultramicroscopy 232: 113395. doi:10.1016/j.ultramic.2021.113395. ISSN 0304-3991. PMID 34653891.
- ↑ Verchenko, Valeriy Yu.; Wei, Zheng; Tsirlin, Alexander A.; Callaert, Carolien; Jesche, Anton; Hadermann, Joke; Dikarev, Evgeny V.; Shevelkov, Andrei V. (2017). "Crystal Growth of the Nowotny Chimney Ladder Phase Fe2Ge3: Exploring New Fe-Based Narrow-Gap Semiconductor with Promising Thermoelectric Performance". Chemistry of Materials 29 (23): 9954–9963. doi:10.1021/acs.chemmater.7b03300. ISSN 0897-4756.
 |