Chemistry:Iron tris(dimethyldithiocarbamate)
| |
| |
| Names | |
|---|---|
| IUPAC name
Tris(dimethyldithiocarbamato)iron
| |
| Other names
Ferric dimethyl dithiocarbamate, Ferbam
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3077, 2771 |
| |
| |
| Properties | |
| [(CH3)2NCS2]3Fe | |
| Molar mass | 416.5 g/mol |
| Appearance | Dark brown to black, odorless solid[1] |
| Density | 1.52 g/cm3 |
| Melting point | Decomposes above 180 °C (356 °F)[1] |
| Boiling point | Decomposes[1] |
| 0.01% (20 °C)[1] | |
| Hazards | |
| Main hazards | Reacts with strong oxidizers, moisture[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
3000 mg/kg (rabbit, oral) 2000 mg/kg (guinea pig, oral) 1130 mg/kg (rat, oral) 3400 mg/kg (mouse, oral)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 15 mg/m3[1] |
REL (Recommended)
|
TWA 10 mg/m3[1] |
IDLH (Immediate danger)
|
800 mg/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide.[3]
Synthesis, structure, bonding
Iron tris(dithiocarbamate)s are typically are prepared by salt metathesis reactions.[3]
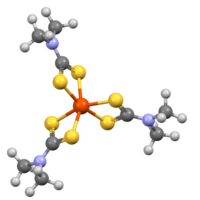
Iron tris(dimethyldithiocarbamate) is an octahedral coordination complex of iron(III) with D3 symmetry.[4]
Spin crossover (SCO) was first observed in 1931 by Cambi et al. who discovered anomalous magnetic behavior for the tris(N,N-dialkyldithiocarbamatoiron(III) complexes.[5] The spin states of these complexes are sensitive to the nature of the amine substituents.[6]
Reactions
Iron tris(dithiocarbamate)s react with nitric oxide to give a nitrosyl complex:
- Fe(dtc)
3 + NO → Fe(dtc)
2NO + 0.5 (dtc)
2
This efficient chemical trapping reaction provides a means to detect NO.[7]
Reflecting the strongly donating properties of dithiocarbamate ligands, iron tris(dithiocarbamate)s oxidize at relatively mild potentials to give isolable iron(IV) derivatives [Fe(S2CNR2)3]+.[8]
Iron tris(dithiocarbamate)s react with hydrochloric acid to give the pentacoordinate chloride:[9]
- Fe(dtc)
3 + HCl → Fe(dtc)
2Cl + Hdtc
Safety
The U.S. Occupational Safety and Health Administration (OSHA) has set the legal (permissible exposure limit) for ferbam exposure in the workplace as 15 mg/m3 over an 8-hour workday. The U.S. National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 1 mg/m3 over an 8-hour workday. At levels of 800 mg/m3, ferbam is immediately dangerous to life and health.[1]
See also
- Zinc dimethyldithiocarbamate - a related dimethyldithiocarbamate complex of zinc
- Nickel bis(dimethyldithiocarbamate) - a related dimethyldithiocarbamate complex of nickel
- Iron tris(diethyldithiocarbamate) - a related dimethyldithiocarbamate complex of iron
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 NIOSH Pocket Guide to Chemical Hazards. "#0286". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0286.html.
- ↑ "Ferbam". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/14484641.html.
- ↑ 3.0 3.1 D. Coucouvanis (2007). "The Chemistry of the Dithioacid and 1,1-Dithiolate Complexes". Progress in Inorganic Chemistry 11: 233–371. doi:10.1002/9780470166123.ch4. ISBN 978-0-470-16612-3.
- ↑ J. Albertsson; Å. Oskarsson (1977). "Compounds with intermediate spin. I. The crystal structure of tris(N,N-dimethyldithiocarbamato)iron(III) at 150 and 295 K". Acta Crystallographica Section B 33 (6): 1871–1877. doi:10.1107/s0567740877007237.
- ↑ L. Cambi; L and L. Szego (1931). "Über die magnetische Susceptibilität der komplexen Verbindungen". Chem. Ber. Dtsch. Ges. 64 (10): 2591. doi:10.1002/cber.19310641002.
- ↑ P. Gütlich; H.A. Goodwin (2004). Spin Crossover in Transition Metal Compounds I. Springer Berlin. ISBN 978-3-540-40396-8.
- ↑ Fujii, S.; Yoshimura, T. (2000). "A New Trend in Iron–Dithiocarbamate Complexes: as an Endogenous NO Trapping Agent". Coordination Chemistry Reviews 198: 89–99. doi:10.1016/S0010-8545(99)00196-4.
- ↑ Pasek, E. A.; Straub, D. K. (1972). "Tris(N,N-Disubstituted Dithiocarbamato)iron(IV) Tetrafluoroborates". Inorganic Chemistry 11 (2): 259–263. doi:10.1021/ic50108a012.
- ↑ Martin, R. L.; White, A. H. (1967). "A Novel Series of fFive-Coordinated Iron(III) Complexes with the Square-Pyramidal Configuration and Spin, S = 3/2". Inorganic Chemistry 6 (4): 712–717. doi:10.1021/ic50050a016.
 |

