Chemistry:Silylene
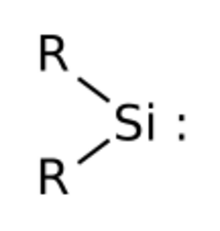 Simplest silylene has R=Hydrogen
| |
| |
| Names | |
|---|---|
| IUPAC name
Silylene
| |
| Systematic IUPAC name
Silylidene[1] | |
| Other names
Hydrogen silicide(−II)
Silicene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| H2Si | |
| Molar mass | 30.101 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Silylene is a chemical compound with the formula SiH2. It is the silicon analog of methylene, the simplest carbene. Silylene is a stable molecule as a gas but rapidly reacts in a bimolecular manner[clarification needed] when condensed. Unlike carbenes, which can exist in the singlet or triplet state, silylene (and all of its derivatives) are singlets.
Silylenes are formal derivatives of silylene with its hydrogens replaced by other substituents.[2] Most examples feature amido (NR2) or alkyl/aryl groups.[3][4] Silylenes have been proposed as reactive intermediates. They are carbene analogs.[5]
Synthesis and properties
Silylenes are generally synthesized by thermolysis or photolysis of polysilanes, by silicon atom reactions (insertion, addition or abstraction), by pyrolysis of silanes, or by reduction of 1,1-dihalosilane. It has long been assumed that the conversion of metallic Si to tetravalent silicon compounds proceeds via silylene intermediates:
- Si + Cl2 → SiCl2
- SiCl2 + Cl2 → SiCl4
Similar considerations apply to the direct process, the reaction of methyl chloride and bulk silicon.
Early observations of silylenes involved generation of dimethylsilylene by dechlorination of dimethyldichlorosilane:[6]
- SiCl2(CH3)2 + 2 K → Si(CH3)2 + 2 KCl
The formation of dimethylsilylene was demonstrated by conducting the dechlorination in the presence of trimethylsilane, the trapped product being pentamethyldisilane:
- Si(CH3)2 + HSi(CH3)3 → (CH3)2Si(H)−Si(CH3)3
A room-temperature isolable N-heterocyclic silylene is N,N′-di-tert-butyl-1,3-diaza-2-silacyclopent-4-en-2-ylidene, first described in 1994 by Michael K. Denk et al.[7]
The α-amido centers stabilize silylenes by π-donation. The dehalogenation of diorganosilicon dihalides is a widely exploited.[8]
Related reactions
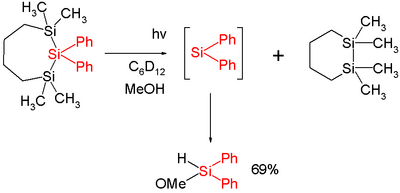
In one study diphenylsilylene is generated by flash photolysis of a trisilane:[9]
In this reaction diphenylsilylene is extruded from the trisila ring. The silylene can be observed with UV spectroscopy at 520 nm and is short-lived with a chemical half-life of two microseconds. Added methanol acts as a chemical trap with a second order rate constant of 1.3×1010 mol−1 s−1 which is close to diffusion control.
See also
- Carbene analogs
- N-heterocyclic silylene
- Silenes, R2Si=SiR2
- Silylium ions, protonated silylenes
References
- ↑ IUPAC Chemical Nomenclature and Structure Representation Division (2013). "P-71.2.2.1". in Favre, Henri A.; Powell, Warren H.. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. IUPAC–RSC. ISBN 978-0-85404-182-4. https://pubs.rsc.org/en/Content/eBook/978-0-85404-182-4.
- ↑ Mizuhata, Yoshiyuki; Sasamori, Takahiro; Tokitoh, Norihiro (2009). "Stable Heavier Carbene Analogues". Chemical Reviews 109 (8): 3479–3511. doi:10.1021/cr900093s. PMID 19630390.
- ↑ 3.0 3.1 Nagendran, Selvarajan; Roesky, Herbert W. (2008). "The Chemistry of Aluminum(I), Silicon(II), and Germanium(II)". Organometallics 27 (4): 457–492. doi:10.1021/om7007869.
- ↑ Haaf, Michael; Schmedake, Thomas A.; West, Robert (2000). "Stable Silylenes". Accounts of Chemical Research 33 (10): 704–714. doi:10.1021/ar950192g. PMID 11041835.
- ↑ Gaspar, Peter; West, R. (1998). "Silylenes". The Chemistry of Organic Silicon Compounds. The Chemistry of Functional Groups. 2. pp. 2463–2568. doi:10.1002/0470857250.ch43. ISBN 0471967572.
- ↑ Skell, P. S.; Goldstein, E. J. (1964). "Dimethylsilene: CH3SiCH3". Journal of the American Chemical Society 86 (7): 1442–1443. doi:10.1021/ja01061a040.
- ↑ Denk, Michael; Lennon, Robert; Hayashi, Randy; West, Robert; Belyakov, Alexander V.; Verne, Hans P.; Haaland, Arne; Wagner, Matthias et al. (1994). "Synthesis and Structure of a Stable Silylene". Journal of the American Chemical Society 116 (6): 2691–2692. doi:10.1021/ja00085a088.
- ↑ Driess, Matthias; Yao, Shenglai; Brym, Markus; Van Wüllen, Christoph; Lentz, Dieter (2006). "A New Type of N-Heterocyclic Silylene with Ambivalent Reactivity". Journal of the American Chemical Society 128 (30): 9628–9629. doi:10.1021/ja062928i. PMID 16866506.
- ↑ Moiseev, Andrey G.; Leigh, William J. (2006). "Diphenylsilylene". Journal of the American Chemical Society 128 (45): 14442–14443. doi:10.1021/ja0653223. PMID 17090011.
 |