Biology:Anomalocaris
| Anomalocaris | |
|---|---|
| |
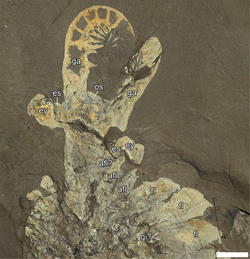
| ROMIP 51212, a largely complete specimen of Anomalocaris canadensis. | |

| |
| Life restoration of Anomalocaris canadensis. | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | †Dinocaridida |
| Order: | †Radiodonta |
| Family: | †Anomalocarididae |
| Genus: | †Anomalocaris Whiteaves, 1892 |
| Species | |
(8 more unnamed species[3]) | |
Anomalocaris ("unlike other shrimp", or "abnormal shrimp") is an extinct genus of radiodont, an order of early-diverging stem-group arthropods.
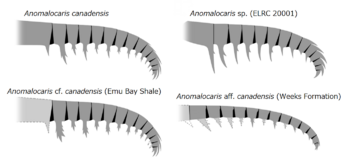
It is best known from the type species A. canadensis, found in the Stephen Formation (particularly the Burgess Shale) of British Columbia, Canada . The species A. daleyae is known from the somewhat older Emu Bay Shale of Australia .[2] Other undescribed remains are known from China and the United States .[3]
Like other radiodonts, Anomalocaris had swimming flaps running along its body, large compound eyes, and a single pair of segmented, frontal appendages, which in Anomalocaris were used to grasp prey. Measuring up to 38 cm (1.25 ft) long excluding frontal appendages and tail fan,[4] A. canadensis is one of the largest animals of the Cambrian, and thought to be one of the earliest examples of an apex predator,[5][6] though others have been found in older Cambrian lagerstätten deposits.
Since the original description in late 19th century,[7] the frontal appendages were the only known fossilized parts and misidentified as the body parts of other animals.[8] Its radiodont affinity was revealed in 1980s, specifically in a 1985 journal article by Harry B. Whittington and Derek Briggs.[9] The trunk and mouth were reconstructed after another radiodont genus until the corrections done in 1996[8] and 2012.[10] It is the type genus of Anomalocarididae, a family which previously included all radiodonts but recently only Anomalocaris and a few closely-related taxa.[3]
Discovery and identification
From the start, Anomalocaris fossil was misidentified, followed by a series of misidentifications and taxonomic revisions.[9][8] As Stephen Jay Gould, who popularised the Cambrian explosion in his 1989 book Wonderful Life, appropriately described:
[The story of Anomalocaris is] a tale of humor, error, struggle, frustration, and more error, culminating in an extraordinary resolution that brought together bits and pieces of three "phyla" in a singe reconstructed creature, the largest and fiercest of Cambrian organisms.[11]
Anomalocaris fossils were first collected in 1886[8] by Richard G. McConnell of the Geological Survey of Canada (GSC). Having been informed of rich fossils at the Stephen Formation in British Columbia, McConnell climbed Mount Stephen on 13 September 1886.[12][13] He found abundant trilobites, along with two unknown specimens.[7] In August 1891, Henri-Marc Ami, Assistant Palaeontologist at GSC, collected many trilobites and brachiopod fossils,[14] along with 48 more of the unknown specimens.[15] The fifty specimens were examined and described in 1892 by GSC paleontologist Joseph Frederick Whiteaves.[16][17] Whiteaves interpreted them as the abdomens of phyllocarid crustaceans, and gave the full scientific name Anomalocaris canadensis. He describes the crustacean characters:
Body or abdominal segments, which, in all the specimens collected, are abnormally flattened laterally, a little higher or deeper than long, broader above than below, the pair of ventral appendages proceeding from each, nearly equal in height or depth to the segment itself... The generic name Anomalocaris (from ανώμαλος, unlike,—καρίς, a shrimp, i.e., unlike other other shrimps) [the species name referring to Canada] is suggested by the unusual shape of the uropods or ventral appendages of the body segments and the relative position of the caudal spine.[7]
In 1928, Danish paleontologist Kai Henriksen proposed that Tuzoia, a Burgess Shale arthropod which was known only from the carapace, represented the missing front half of Anomalocaris.[8] The artists Elie Cheverlange and Charles R. Knight followed this interpretation in their depictions of Anomalocaris.[8]
Not known to scientists at the time, the body parts of relatives of Anomalocaris had already been described but not recognized as such. The first fossilized mouth of such a kind of animal was discovered by Charles Doolittle Walcott, who mistook it for a jellyfish and placed it in the genus Peytoia. Walcott also discovered a frontal appendage but failed to realize the similarities to Whiteaves' discovery and instead identified it as feeding appendage or tail of the coexisted Sidneyia.[18] In the same publication in which he named Peytoia, Walcott named Laggania, a taxon that he interpreted as a holothurian.
In 1966, the Geological Survey of Canada began a comprehensive revision of the Burgess Shale fossil record, led by Cambridge University paleontologist Harry B. Whittington.[8] In the process of this revision, Whittington and his students Simon Conway Morris and Derek Briggs would discover the true nature of Anomalocaris and its relatives, but not without contributing to the history of misinterpretations first.[18] In 1978, Conway Morris recognized that the mouthparts of Laggania were identical to Peytoia, but concluded that Laggania was a composite fossil made up of Peytoia and the sponge Corralio undulata.[19] In 1979, Briggs recognized that the fossils of Anomalocaris were appendages, not abdomens, and proposed that they were the walking legs of a giant arthropod, and that the feeding appendage Walcott had assigned to Sidneyia was the feeding appendage of similar animal, referred to as "appendage F".[20] Later, while clearing what he thought was an unrelated specimen, Harry B. Whittington removed a layer of covering stone to discover the unequivocally connected frontal appendage identical to Anomalocaris and mouthpart similar to Peytoia.[18][21] Whittington linked the two species, but it took several more years for researchers to realize that the continuously juxtaposed Peytoia, Laggania and frontal appendages (Anomalocaris and "appendage F") actually represented a single group of enormous creatures.[9] The two genera have now been placed into the order Radiodonta[8] and are commonly known as radiodonts or anomalocaridids. Since Peytoia was named first, it is the accepted correct name for the entire animal. However, the original frontal appendage was from a larger species distinct from Peytoia and "Laggania" and therefore retains the name Anomalocaris.[10]
In 2011[22] and 2020,[23] compound eyes of Anomalocaris were recovered from a paleontological dig at Emu Bay on Kangaroo Island, Australia, proving that Anomalocaris was indeed an arthropod as had been suspected. The find also indicated that advanced arthropod eyes had evolved very early, before the evolution of jointed legs or hardened exoskeletons.[22]
In 2021, "A." saron[24] and "A." magnabasis[25] were reassigned to the new genus Houcaris, in the family Tamisiocarididae.[26] In the same year, "A." pennsylvanica was reassigned to the genus Lenisicaris.[3] In 2022, specimen ELRC 20001 that was treated as an unnamed species of Anomalocaris or whole-body specimen of A. saron got a new genus, Innovatiocaris.[27] In 2023, "A". kunmingensis was reassigned to the new genus Guanshancaris in the family Amplectobeluidae.[28] Multiple phylogenetic analyses also suggested that "A". briggsi (tamisiocaridid) was not a species of Anomalocaris either,[29][30][31][4][32][33][34] and it was reassigned to the genus Echidnacaris in the family Tamisiocarididae in 2023.[2] In the same 2023 study, a new species of Anomalocaris, A. daleyae, was described based on remains found in the Emu Bay Shale in Australia.[2]
Description
For the time in which it lived, Anomalocaris was gigantic. A. canadensis is estimated to be up to 37.8 cm (1.24 ft) long excluding frontal appendages and tail fans.[4][27] Previous estimation up to 1 m (3.3 ft)[20] is unlikely based on the ratio of body parts (body length measured only about 2 times the length of frontal appendage in A. canadensis, respectively[4]) and the size of largest frontal appendage (up to 18 centimetres (7.1 inches) in length when extended).[18][4]
Anomalocaris propelled itself through the water by undulating the flexible flaps on the sides of its body.[35] Each flap sloped below the one more posterior to it,[36] and this overlapping allowed the lobes on each side of the body to act as a single "fin", maximizing the swimming efficiency.[35] The construction of a remote-controlled model showed this mode of swimming to be intrinsically stable,[37] implying that Anomalocaris would not have needed a complex brain to manage balance while swimming. The body was widest between the third and fifth lobe and narrowed towards the tail, with additional 3 pairs of small flaps on the constricted neck region.[8][38] It is difficult to distinguish lobes near the tail, making an accurate count difficult.[36] For the main trunk flaps, the type species A. canadensis had 13 pairs.[38]
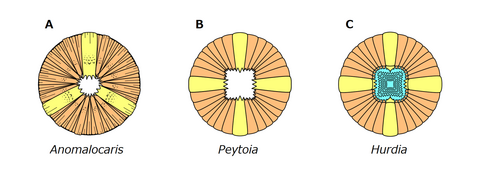
Oral cones of Anomalocaris (A), Peytoia (B) and Hurdia (C), the former showing unique triradial, tuberculated and wrinkled structures.
The head sclerite structure of Anomalocaris.
Anomalocaris had an unusual disk-like mouth known as oral cone. The oral cone was composed of several plates organized triradially. Three of the plates were quite large. Three to four medium sized plates could be found between each of the large plates, and several small plates between them. Most of the plates wrinkled and have scale-like tubercles near the mouth opening.[10][39] Such an oral cone is very different from those of a typical hurdiid radiodont like Peytoia and Hurdia, which is smooth and tetraradial.[10][33] As a shared character across radiodonts, Anomalocaris also had three sclerites on the top and side of its head.[33] The top one, known as a head shield, dorsal carapace or H-element, was shaped like an laterally-elongated[40] oval, with a distinct rim on the outer edge.[38] The remaining two lateral sclerites, known as P-elements, were also ovoid, but connected by a bar-like outgrowth.[33] The P-elements were previously misinterpreted as two huge compound eyes.[38][33]
Two large frontal appendage were positioned in front of the mouth, at the front of the head.[8] Each frontal appendage of Anomalocaris usually had 14 podomeres (segmental units, at least 1 for shaft and 13 for distal articulated region), with each appendage being laterally-flattened (taller than wide).[38] Most podomeres were tipped with a pair of endites (ventral spines).[38] The endites themselves were both equipped with multiple auxiliary spines, which branches off from the anterior and posterior margin of the endites.[24][39][41][38][1]
The tail was a large tail fan, composed of three[8][38] pairs of large, lateral fin-shaped lobes and one terminal lobe-like tailpiece.[38] Previous studies suggest the tail fan was used to propel it through Cambrian waters,[21][35] while further hydrodynamic study rather suggest it was more adapted to provide steering function.[42] The gills of the animal, in the form of long, thin, hair-like structures known as lanceolate blades, were arranged in rows forming setal blades. The setal blades were attached by their margin to the top side of the animal, two setal blades per body segment. A divide ran down the middle, separating the gills.[38]
Based on fossilized eyes from the Emu Bay Shale, which belong to the species Anomalocaris daleyae,[2] the stalked eyes of Anomalocaris were 30 times more powerful than those of trilobites, long thought to have had the most advanced eyes of any contemporary species. With one specimen having over 24,000 lenses in one eye, the resolution of the 3-centimetre-wide (1.2 in) eyes would have been rivalled only by that of the modern dragonfly, which has 28,000 lenses in each eye.[22][23] Additionally, estimation of ecdysozoan opsins suggest that Anomalocaris may have had dichromatic color vision.[43]
Paleobiology
Diet
The interpretation of Anomalocaris as an active predator is widely accepted throughout the history of research,[9][8][10] as its raptorial frontal appendages and mid-gut glands strongly suggest a predatory lifestyle.[44][45][5] In the case of A. canadensis, its outstanding size amongst Burgess Shale fauna also make it one of the first apex predators known to exist.[5]
However, the long-standing idea that Anomalocaris fed on hard-bodied animals, especially its ability to penetrate mineralized exoskeleton of trilobites, has been questioned, with many recent studies considering it more likely that Anomalocaris exclusively hunted soft-bodied prey.[46][10][5][6] Some Cambrian trilobites have been found with round or W-shaped "bite" marks, which were identified as being the same shape as the mouthparts of Peytoia (previously misidentified as those of Anomalocaris[47][10]). Stronger evidence that Anomalocaris ate trilobites comes from coprolite, which contain trilobite parts and are so large that the radiodonts are the only known organism from that period large enough to have produced them.[47] However, since Anomalocaris lacks any mineralized tissue, it seemed unlikely that it would be able to penetrate the hard, calcified exoskeleton of trilobites.[47] Rather, the coprolites may have been produced by different organisms, such as the trilobites of the genus Redlichia.[39] Another suggested possibility was that Anomalocaris fed by grabbing one end of their prey in its oral cone while using its frontal appendages to quickly rock the other end of the animal back and forth. This produced stresses that exploited the weaknesses of arthropod cuticles, causing the prey's exoskeleton to rupture and allowing the predator to access its innards.[47] This behaviour was originally thought to have provided an evolutionary pressure for trilobites to roll up, to avoid being flexed until they snapped.[47]

The lack of wear on radiodont mouthparts suggests they did not come into regular contact with mineralized trilobite shells, and were possibly better suited to feeding on smaller, soft-bodied organisms by suction, since they would have experienced structural failure if they were used against the armour of trilobites.[46][39] A. canadensis was suggested to have been capable of feeding on organisms with hard exoskeletons due to the short, robust spines on its frontal appendages.[39][25] However, this conclusion is solely based on the comparison with the fragile frontal appendages of suspension feeding radiodonts (e.g. Echidnacaris and Houcaris spp.).[26] The typical lack of damage to the endites on the frontal appendages of A. canadensis (with damage only present on a single specimen) suggests that they were not used to grasp hard-shelled prey.[6] As opposed to Peytoia whose oral cone is more rectangular with short protruding spines, the oral cone of A. canadensis has a smaller and more irregular opening, not permitting strong biting motions, and indicating a suction-feeding behavior to suck in softer organisms.[10] Three-dimensional modelling of various radiodont frontal appendages also suggest that A. canadensis is more capable to prey on smaller (2–5 cm in diameter), active, soft-bodied animals (e.g. vetulicolian; free-swimming arthropods like isoxyids and hymenocarines; Nectocaris).[5][6]
Bicknell et al. (2023) examined the frontal appendages of Anomalocaris, suggesting it was an active nektonic apex predator. Postured with the frontal appendages outstretched, Anomalocaris would have been able to swim with maximized speed, similar to modern predatory water bugs. Its eyes would be suitable to hunt prey in well-lit waters. Anomalocaris would have hunted various free-swimming animals since there are a large diversity of nektonic and pelagic soft-bodied animals. It probably would have not hunted benthic animals like trilobites, considering the possibility of damaging the frontal appendages on the substrate while trying to grab prey from seafloor at speed. Instead, other animals such as other radiodonts (e.g. Hurdia, Cambroraster, Titanokorys, Stanleycaris) and artiopods (e.g. Sidneyia) would have been benthic predators in the Burgess Shale.[5][6]
Paleoecology
Specimens of Anomalocaris have been found worldwide spanning from Cambrian Stage 3 to the Guzhangian. Aside from the Burgess Shale and Emu Bay Shale, fossils have been found in the Chengjiang Biota, Hongjingshao Formation, Balang Formation and the Kaili Formation of China, as well as the Eagar Formation and Weeks Formation in the United States.[3]
Anomalocaris canadensis lived in the Burgess Shale in relatively great numbers.[1] In the Burgess Shale, Anomalocaris is more common in the older sections, notably the Mount Stephen trilobite beds. However, in the younger sections, such as the Phyllopod bed, Anomalocaris could reach much greater sizes; roughly twice the size of its older, trilobite bed relatives. These rare giant specimens have previously been referred to a separate species, Anomalocaris gigantea; however, the validity of this species has been called into question,[20] and is currently synonymized to A. canadensis.[38]
Other unnamed species of Anomalocaris live in vastly different environments.[3] For example, Anomalocaris cf. canadensis (JS-1880) lived in the Maotianshan Shales,[3] a shallow tropical sea or even being delta[48] in what is now modern China. Anomalocaris daleyae (Emu Bay Shale) lived in a comparable environment; the shallow, tropical waters of Cambrian Australia.[3] The Maotianshan Shale and the Emu Bay Shale are very close in proximity, being separated by a small landmass, far from the Burgess Shale.[3] These two locations also included "Anomalocaris" kunmingensis and "Anomalocaris" briggsi respectively, species that previously attributed[49][50][39][51] but taxonomically unlikely to be a member of Anomalocaris nor even Anomalocarididae.[3][52]
See also
- 8564 Anomalocaris, an asteroid named after this animal.
- Radiodonta, extinct arthropod order composed of Anomalocaris and its relatives.
- Houcaris, Lenisicaris, Innovatiocaris, Guanshancaris, Echidnacaris, radiodont genera containing species originally named as Anomalocaris.
- Aegirocassis, a giant filter-feeding radiodont from Ordovician Morocco.
- Cambrian explosion, the large bio-diversification event that occurred during the Cambrian.
- Opabinia, a genus of bizarre stem-group arthropod distantly related to the radiodonts.
- Wiwaxia, a genus of possible mollusk that had copious numbers of carbonaceous scales, and lived alongside Anomalocaris.
- Paleobiota of the Burgess Shale
Footnotes
- ↑ 1.0 1.1 1.2 "Arthropod appendages from the Weeks Formation Konservat-Lagerstätte: new occurrences of anomalocaridids in the Cambrian of Utah, USA" (in en). Bulletin of Geosciences: 269–282. 2014-05-19. doi:10.3140/bull.geosci.1442. http://www.geology.cz/bulletin/contents/art1442.
- ↑ 2.0 2.1 2.2 2.3 2.4 Paterson, John R.; García-Bellidob, Diego C.; Edgecombe, Gregory D. (10 July 2023). "The early Cambrian Emu Bay Shale radiodonts revisited: morphology and systematics". Journal of Systematic Palaeontology 21 (1). doi:10.1080/14772019.2023.2225066. https://www.tandfonline.com/doi/full/10.1080/14772019.2023.2225066.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 "New anomalocaridids (Panarthropoda: Radiodonta) from the lower Cambrian Chengjiang Lagerstätte: Biostratigraphic and paleobiogeographic implications". Palaeogeography, Palaeoclimatology, Palaeoecology 569: Article 110333. 2021. doi:10.1016/j.palaeo.2021.110333. Bibcode: 2021PPP...569k0333W.
- ↑ 4.0 4.1 4.2 4.3 4.4 "New suspension-feeding radiodont suggests evolution of microplanktivory in Cambrian macronekton". Nature Communications 9 (1): 3774. September 2018. doi:10.1038/s41467-018-06229-7. PMID 30218075. Bibcode: 2018NatCo...9.3774L.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "Three-dimensional modelling, disparity and ecology of the first Cambrian apex predators". Proceedings. Biological Sciences 288 (1955): 20211176. July 2021. doi:10.1098/rspb.2021.1176. PMID 34284622.
- ↑ 6.0 6.1 6.2 6.3 6.4 "Raptorial appendages of the Cambrian apex predator Anomalocaris canadensis are built for soft prey and speed" (in en). Proceedings of the Royal Society B: Biological Sciences 290 (2002). 2023-07-12. doi:10.1098/rspb.2023.0638. ISSN 0962-8452. PMID 37403497.
- ↑ 7.0 7.1 7.2 "Description of a new genus and species of phyllocarid Crustacea from the Middle Cambrian of Mount Stephen, B.C.". Canadian Record of Science 5 (4): 205–208. 1892. https://books.google.com/books?id=gEcbAAAAYAAJ.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 "The "Evolution" of Anomalocaris and Its Classification in the Arthropod Class Dinocarida (nov.) and Order Radiodonta (nov.)". Journal of Paleontology 70 (2): 280–293. 1996. doi:10.1017/S0022336000023362. Bibcode: 1996JPal...70..280C.
- ↑ 9.0 9.1 9.2 9.3 "The largest Cambrian animal, Anomalocaris, Burgess Shale, British Columbia". Philosophical Transactions of the Royal Society B 309 (1141): 569–609. 1985. doi:10.1098/rstb.1985.0096. Bibcode: 1985RSPTB.309..569W.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 "The oral cone of Anomalocaris is not a classic peytoia". Die Naturwissenschaften 99 (6): 501–4. June 2012. doi:10.1007/s00114-012-0910-8. PMID 22476406. Bibcode: 2012NW.....99..501D. https://www.academia.edu/1517990.
- ↑ Wonderful Life: The Burgess Shale and the Nature of History. New York: W.W. Norton. 1989. p. 194. ISBN 0-393-02705-8. OCLC 18983518. https://www.worldcat.org/oclc/18983518.
- ↑ "The Burgess Shale: First Discoveries" (in en-US). Royal Ontario Museum. https://burgess-shale.rom.on.ca/history/discoveries/.
- ↑ "How the Burgess Shale came to Cambridge; and what happened", Cambridge Minds (Cambridge University Press): pp. 126–141, 1994-09-01, doi:10.1017/cbo9780511523007.011, ISBN 978-0-521-45405-6, https://www.cambridge.org/core/product/identifier/CBO9780511523007A016/type/book_part, retrieved 2023-03-25
- ↑ (in en) Catalogue of a Stratigraphical Collection of Canadian Rocks Prepared for the World's Columbian Exposition, Chicago, 1893. Ottawa (Canada): Geological Survey of Canada/Government Printing Bureau. 1893. p. 87. ISBN 9781014253446. https://books.google.com/books?id=HVHPAAAAMAAJ&dq=ami+1891+mount+stephen&pg=PA87.
- ↑ "I.—The Canadian Rockies. Part I: On a Collection of Middle Cambrian Fossils obtained by Edward Whymper, Esq., F.R.G.S., from Mount Stephen, British Columbia" (in en). Geological Magazine 9 (12): 529–544. 1902. doi:10.1017/S001675680018149X. ISSN 0016-7568. Bibcode: 1902GeoM....9..529W. https://www.cambridge.org/core/product/identifier/S001675680018149X/type/journal_article.
- ↑ "Anomalocaris, the largest known Cambrian arthropod". Palaeontology 22 (3): 631–664. 1979. https://www.palass.org/publications/palaeontology-journal/archive/22/3/article_pp631-664.
- ↑ "Description of a new genus and species of phyllocarid Crustacea from the Middle Cambrian of Mount Stephen, B. C.". The Canadian Record of Science 5 (4). 1892.
- ↑ 18.0 18.1 18.2 18.3 Wonderful life: the Burgess Shale and the nature of history. New York: W.W. Norton. 1989. pp. 194–206. ISBN 978-0-393-02705-1.
- ↑ "Laggania cambria Walcott: A Composite Fossil". Journal of Paleontology 52 (1): 126–131. 1978.
- ↑ 20.0 20.1 20.2 "Anomalocaris, the largest known Cambrian arthropod". Palaeontology 22 (3): 631–664. 1979.
- ↑ 21.0 21.1 The crucible of creation: the Burgess Shale and the rise of animals. Oxford [Oxfordshire]: Oxford University Press. 1998. pp. 56–9. ISBN 978-0-19-850256-2.
- ↑ 22.0 22.1 22.2 "Acute vision in the giant Cambrian predator Anomalocaris and the origin of compound eyes". Nature 480 (7376): 237–40. December 2011. doi:10.1038/nature10689. PMID 22158247. Bibcode: 2011Natur.480..237P. https://www.researchgate.net/publication/51868241.
- ↑ 23.0 23.1 "Disparate compound eyes of Cambrian radiodonts reveal their developmental growth mode and diverse visual ecology". Science Advances 6 (49): eabc6721. December 2020. doi:10.1126/sciadv.abc6721. PMID 33268353. Bibcode: 2020SciA....6.6721P.
- ↑ 24.0 24.1 "Anomalocaris and other large animals in the lower Cambrian Chengjiang fauna of southwest China". GFF 117 (3): 163–183. 1995-09-01. doi:10.1080/11035899509546213. ISSN 1103-5897.
- ↑ 25.0 25.1 "Systematics, preservation and biogeography of radiodonts from the southern Great Basin, USA, during the upper Dyeran (Cambrian Series 2, Stage 4)" (in en). Papers in Palaeontology 7: 235–262. 2019. doi:10.1002/spp2.1277. ISSN 2056-2799. https://serval.unil.ch/notice/serval:BIB_29DFB7FB5010.
- ↑ 26.0 26.1 "Houcaris gen. nov. from the early Cambrian (Stage 3) Chengjiang Lagerstätte expanded the palaeogeographical distribution of tamisiocaridids (Panarthropoda: Radiodonta)". PalZ 95 (2): 209–221. 2021. doi:10.1007/s12542-020-00545-4. ISSN 1867-6812.
- ↑ 27.0 27.1 "Innovatiocaris, a complete radiodont from the early Cambrian Chengjiang Lagerstätte and its implications for the phylogeny of Radiodonta". Journal of the Geological Society 180. 2022-09-07. doi:10.1144/jgs2021-164. ISSN 0016-7649.
- ↑ "Amplectobeluid Radiodont Guanshancaris gen. nov. from the Lower Cambrian (Stage 4) Guanshan Lagerstätte of South China: Biostratigraphic and Paleobiogeographic Implications". Biology 12 (4): 583. April 2023. doi:10.3390/biology12040583. PMID 37106783.
- ↑ "A suspension-feeding anomalocarid from the Early Cambrian". Nature 507 (7493): 496–9. March 2014. doi:10.1038/nature13010. PMID 24670770. Bibcode: 2014Natur.507..496V. http://dro.dur.ac.uk/21270/1/21270.pdf.
- ↑ "Brain structure resolves the segmental affinity of anomalocaridid appendages". Nature 513 (7519): 538–42. September 2014. doi:10.1038/nature13486. PMID 25043032. Bibcode: 2014Natur.513..538C.
- ↑ "Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps". Nature 522 (7554): 77–80. June 2015. doi:10.1038/nature14256. PMID 25762145. Bibcode: 2015Natur.522...77V.
- ↑ "Origin of raptorial feeding in juvenile euarthropods revealed by a Cambrian radiodontan". National Science Review 5 (6): 863–869. 2018-11-01. doi:10.1093/nsr/nwy057. ISSN 2095-5138.
- ↑ 33.0 33.1 33.2 33.3 33.4 "A new hurdiid radiodont from the Burgess Shale evinces the exploitation of Cambrian infaunal food sources". Proceedings. Biological Sciences 286 (1908): 20191079. August 2019. doi:10.1098/rspb.2019.1079. PMID 31362637.
- ↑ "Exceptional multifunctionality in the feeding apparatus of a mid-Cambrian radiodont" (in en). Paleobiology 47 (4): 704–724. 2021. doi:10.1017/pab.2021.19. ISSN 0094-8373. Bibcode: 2021Pbio...47..704M. https://zenodo.org/record/4682069.
- ↑ 35.0 35.1 35.2 "Theoretical study on the body form and swimming pattern of Anomalocaris based on hydrodynamic simulation". Journal of Theoretical Biology 238 (1): 11–7. January 2006. doi:10.1016/j.jtbi.2005.05.008. PMID 16002096. Bibcode: 2006JThBi.238...11U.
- ↑ 36.0 36.1 "The Largest Cambrian Animal, Anomalocaris, Burgess Shale, British Columbia". Philosophical Transactions of the Royal Society B 309 (1141): 569–609. 1985. doi:10.1098/rstb.1985.0096. Bibcode: 1985RSPTB.309..569W.
- ↑ "Giant predators from the Cambrian of China". Science 264 (5163): 1283–4. May 1994. doi:10.1126/science.264.5163.1283. PMID 17780843. Bibcode: 1994Sci...264.1283B.
- ↑ 38.00 38.01 38.02 38.03 38.04 38.05 38.06 38.07 38.08 38.09 38.10 "Morphology of Anomalocaris canadensis from the Burgess Shale". Journal of Paleontology 88 (1): 68–91. January 2014. doi:10.1666/13-067. Bibcode: 2014JPal...88...68D. https://www.academia.edu/6947803.
- ↑ 39.0 39.1 39.2 39.3 39.4 39.5 "New anatomical information on Anomalocaris from the Cambrian Emu Bay Shale of South Australia and a reassessment of its inferred predatory habits". Palaeontology: n/a. 2013. doi:10.1111/pala.12029.
- ↑ "Morphology of diverse radiodontan head sclerites from the early Cambrian Chengjiang Lagerstätte, south-west China". Journal of Systematic Palaeontology 16 (1): 1–37. 2018-01-02. doi:10.1080/14772019.2016.1263685. ISSN 1477-2019. https://figshare.com/articles/dataset/Morphology_of_diverse_radiodontan_head_sclerites_from_the_early_Cambrian_Chengjiang_Lagerst_tte_south-west_China/4516751.
- ↑ "The first discovery of anomalocaridid appendages from the Balang Formation (Cambrian Series 2) in Hunan, China". Alcheringa: An Australasian Journal of Palaeontology 37 (3): 338–343. 2013-09-01. doi:10.1080/03115518.2013.753767. ISSN 0311-5518.
- ↑ "On the Hydrodynamics of Anomalocaris Tail Fins". Integrative and Comparative Biology 58 (4): 703–711. October 2018. doi:10.1093/icb/icy014. PMID 29697774.
- ↑ "Molecular palaeontology illuminates the evolution of ecdysozoan vision". Proceedings. Biological Sciences 285 (1892): 20182180. December 2018. doi:10.1098/rspb.2018.2180. PMID 30518575.
- ↑ "Sophisticated digestive systems in early arthropods". Nature Communications 5 (1): 3641. May 2014. doi:10.1038/ncomms4641. PMID 24785191. Bibcode: 2014NatCo...5.3641V.
- ↑ Reconstructing anomalocaridid feeding appendage dexterity sheds light on radiodontan ecology (Report). 16 December 2016.
- ↑ 46.0 46.1 Template:Walcott2009
- ↑ 47.0 47.1 47.2 47.3 47.4 "Anomalocaris predation on nonmineralized and mineralized trilobites". Geology 27 (11): 987–990. 1999. doi:10.1130/0091-7613(1999)027<0987:APONAM>2.3.CO;2. Bibcode: 1999Geo....27..987N.
- ↑ "The Chengjiang Biota inhabited a deltaic environment". Nature Communications 13 (1): 1569. March 2022. doi:10.1038/s41467-022-29246-z. PMID 35322027. Bibcode: 2022NatCo..13.1569S.
- ↑ Nedin, Christopher (1995). The Emu Bay Shale, a Lower Cambrian fossil Lagerstatte, Kangaroo Island, South Australia.
- ↑ "New anomalocardid frontal appendages from the Guanshan biota, eastern Yunnan" (in en). Chinese Science Bulletin 58 (32): 3937–3942. 2013-11-01. doi:10.1007/s11434-013-5908-x. ISSN 1861-9541. Bibcode: 2013ChSBu..58.3937W. https://www.researchgate.net/publication/257689210.
- ↑ "Mapping the world's Burgess Shale-type deposits" (in EN). http://www.virtualmuseum.ca/edu/ViewLoitDa.do;jsessionid=12EF81081303D31D7A2C8CC0E294A505?method=preview&lang=EN&id=19487.
- ↑ "The endemic radiodonts of the Cambrian Stage 4 Guanshan biota of South China" (in en). Acta Palaeontologica Polonica 66. 2021. doi:10.4202/app.00870.2020. ISSN 0567-7920.
External links
- "Anomalocaris canadensis". Burgess Shale Fossil Gallery. Virtual Museum of Canada. 2011. http://burgess-shale.rom.on.ca/en/fossil-gallery/view-species.php?id=1.
- Anomalocaris 'homepage' with swimming animation
- Burgess Shale: Anomalocaris canadensis (proto-arthropod), Smithsonian.
Wikidata ☰ Q37395 entry
 |