Biology:Tex36
Testis expressed 36, TEX36, is a protein that in humans is encoded by the tex36 gene.[1] TEX36 interacts with proteins involved in the MAP kinase family, supporting that TEX36 may be regulated with on or off configurations.[2] The encoded protein is highly expressed in fetal, testes, and placental tissues and has background expression levels in adults.[3] There are also many motifs specific to male sex determination and spermatogenic factors, suggesting that it is involved in development.[4]
Gene
The verified gene sequence was confirmed in NCBI on November 26, 2016. The coding region spans 106,622 bases, and within that region are 4 exons.
Aliases
TEX36, is also known by the aliases C10orf122 and BA383C5.1.[5] It has cytogenetic bands at 10q26.13. TEX36 is also a member of the Human CCDS set CCDS44493.1.[6]
Locus
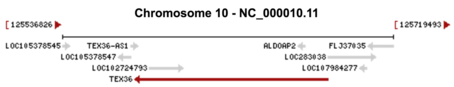
The gene spans from 125576522 to 125683144 in the human genome. Figured is the genomic context of TEX36. There is aldolase, fructose-biphosphate A2 along with several uncharacterized loci in the gene neighborhood of TEX36.[1]
mRNA
There are two variants of TEX36, transcripts 1 and 2. The two differ by an alternately spliced 4th exon. Validated variants, 1 and 2, both contain 4 exons, but variant 1 has a longer transcript with 922 base pairs, whereas variant 2 contains 777 base pairs with a different terminating region. Only variant 1 is the protein encoding transcript.[1]
Protein
Variant 1 encodes protein testis expressed 36. TEX36 does not have any confirmed isoforms.[1] The unmodified TEX36 consists of 186 amino acids and has a molecular weight of 21,545 daltons, with an isoelectric point of 9.1.[5][7] Amino acids serine and lysine are highly represented in the protein at a higher frequency than observed in most proteins in vertebrates.[8]
Domains & Motifs
| Domain | Location | Notes |
|---|---|---|
| VVSS | 136-139 | Repeat[10] |
| HDNR | 73-76 | Domain of unknown function, found in 6 human proteins[11] |
| Pat4 | 167, 168 | Nuclear localization signal[10] |
| MapK | 13-18 | MapK interacting docking motif, overlaps with beta strand[2] |
Secondary Structure
Based on conservation through multiple sequence alignments and multiple secondary structure prediction algorithms, TEX36 is composed of 4 alpha helices and 5 beta strands.[12][13]
| 1 | 2 | 3 | 4 | 5 | |
| Alpha Helix | 47-48 | 57-62 | 148-153 | 161-167 | |
| Beta Strand | 16-21 | 31-33 | 70-72 | 128-140 | 171-184 |
Post-Translational Modifications
14 of the 20 serine residues in TEX36 appear to be post-translationally phosphorylated.[14] There are protein kinase C and casein kinase phosphorylation domains, along with a cAMP phosphodiesterase site and amidation sites.[15] These post translational modifications, specifically phosphorylation, may regulate TEX36 to be off when phosphorylated and in an on conformation when not.[2]
Sub-cellular Localization
There are a couple of nuclear localization signals, pat4, and strong support from other analyses that TEX36 may be localized to the nucleus. There is not strong support for it to be found in the ER, cytoplasm, or vacuoles. These findings were consistent for the human TEX36 along with distant orthologs, such as the sea anemone and canary.
Expression and Regulation
Regulation of Gene Expression
The promoter region contains the following regulatory sequences that are in highly conserved regions of TEX36:
| Sequence | Number |
|---|---|
| Spermatogenic Zip1[16] | 3 |
| Nanog | 1 |
| Sex determining region Y | 1 |
| SRY Box 9 | 2 |
| SRY-related HMG-box 30 | 1 |
Regulation of Translation
In the 5'UTR, there is one stable stem loop, which contains a eukaryotic translation initiation factor motif.[17] There was also a splice factor in the 5'UTR.[17] In both the 5'UTR and 3'UTR are X-linked RNA binding motifs.[17] One of the three stable stem loops in the 3'UTR contained an X-linked RNA binding motif.[2] There were no identified miRNA targets in either untranslated regions.[18]
Tissue Expression
Based on microarray data, TEX36 is highly expressed in placental, fetal, and testis tissues.[3] Whereas remains are more background levels in other adult tissues.[4] Also, TEX36 has shown to have a two-fold increase in expression levels after exercise, compared to before in endothelial progenitor cells.[19]
Function
Interacting Proteins
TEX36 has been shown to interact with:
- Dexamethasone-induced Ras-related protein 1[20]
- phosphatase 1 catalytic subunit[21]
- interferon regulatory factor 3[21]
Variants, Pathology and Clinical Significance
TEX36 is one of 77 proteins expressed by testes capable of interacting with protein phosphatase 1, PP1, human testis protein protein phosphatase 1.[22] One variant of TEX36, p.R142H, was found across all patients in a study on intracranial aneurysms. However, there was no further research on the gene and its protein function.[23] TEX36 has also been found to be a commonly down-regulated gene in patients with microalbuminuria compared to those with normoalbuminuria.[24] Because of the variant associated with intracranial aneurysms and its downregulation associated with various albumin levels, protein changes in TEX36 either in variants or expression levels could be deleterious, but there is no confirmation on these assumptions.[25]
Homology
Paralogs
There are no known paralogs of TEX36.[26]
Ortholog Space

Identified through BLAST and BLAT, species that have contain an ortholog of TEX36, include: mammals, birds, reptiles, amphibians, fish, echinoids, anthozoans, and molluscs.[28][26] There are no confirmed orthologs in bacteria, fungi/metazoans, plants, or archaea.[29] TEX36 is found often in mammals, fish and birds, and more sparse in the others.[29][30]
| Specific Name | Common Name | Order | Accession Number | Divergence Time (MYA) | Length (AA) | Identity | Similarity |
| Homo sapiens | Human | Primates | NP_001121674.1 | 0 | 186 | 100 | 100 |
| Panthera tigris altaica | Siberian Tiger | Carnivora | XP_007079755.1 | 96 | 182 | 71.8 | 80.9 |
| Tursiops truncatus | Bottlenose Dolphin | Artiodactyla | XP_019795781.1 | 96 | 163 | 65.1 | 71.5 |
| Dasypus novemcinctus | Armadillo | Cingulata | XP_004446737.1 | 105 | 181 | 76.9 | 82.8 |
| Buceros rhinoceros silvestris | Rhinoceros hornbill | Bucerotiformes | XP_010142644.1 | 312 | 187 | 42.6 | 57.9 |
| Chrysemys picta bellii | Painted Turtle | Testudines | XP_005281969.1 | 312 | 164 | 38.7 | 54.8 |
| Xenopus laevis | African Clawed Frog | Anura | XP_018082893.1 | 352 | 163 | 31.1 | 42.5 |
| Latimeria chalumnae | Ocean Coelacanth | Coelacanthiformes | XP_014343024.1 | 413 | 183 | 33.8 | 49.3 |
| Strongylocentrotus purpuratus | Purple Sea Urchin | Echinoida | XP_001184205.2 | 684 | 200 | 28.6 | 28.8 |
| Crassostrea gigas | Pacific Oyster | Ostreoida | XP_011437377.1 | 797 | 238 | 23.5 | 33.7 |
| Exaiptasia pallida | Sea Anemone | Actiniaria | KXJ14560.1 | 824 | 182 | 23 | 32 |
Protein Evolution
Compared to a fast evolving protein, fibrinogen factor A, and a slow evolving protein, cytochrome c1, TEX36 has a similar rate of evolution to fibrinogen factor A, suggesting it is a fast evolving protein.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "TEX36 testis expressed 36 [Homo sapiens (Human)] - Gene - NCBI". https://www.ncbi.nlm.nih.gov/gene/387718.
- ↑ 2.0 2.1 2.2 2.3 Ehrenberger, Tobias. "The NEW Scansite 3". http://scansite3.mit.edu/#proteinScan;method=input.
- ↑ 3.0 3.1 Kocabas, A. M., Crosby, J., Ross, P. J., Otu, H. H., Beyhan, Z., Can, H., ... & Fernandez, E. (2006). The transcriptome of human oocytes. Proceedings of the National Academy of Sciences, 103(38), 14027-14032.
- ↑ 4.0 4.1 Group, Schuler. "EST Profile - Hs.148259". https://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist=Hs.148259.
- ↑ 5.0 5.1 GeneCards, Human Gene Database, entry on TEX36, [http://www.genecards.org/cgi-bin/carddisp.pl?gene=TEX36]
- ↑ Ensembl entry on Gene: TEX36, [http://useast.ensembl.org/Homo_sapiens/Gene/Summary?g=ENSG00000175018;r=10:125576522-125683144]
- ↑ "ExPASy - Compute pI/Mw tool" (in en-US). http://web.expasy.org/compute_pi/.
- ↑ Dyer, K. F. 1971. The quiet revolution: A new synthesis of biological knowledge. Journal of Biological Education 5:15-24
- ↑ Castro, Edouard de. "PROSITE" (in en-US). http://prosite.expasy.org/cgi-bin/prosite/mydomains/.
- ↑ 10.0 10.1 Nakai, K. and Horton, P., PSORT: a program for detecting the sorting signals of proteins and predicting their subcellular localization, Trends Biochem. Sci, 24(1) 34-35 (1999).
- ↑ EMBL-EBI, InterPro. "Species: Domain of unknown function with conserved HDNR motif (IPR029369) < InterPro < EMBL-EBI" (in en). http://www.ebi.ac.uk/interpro/entry/IPR029369/taxonomy.
- ↑ 12.0 12.1 "I-TASSER server for protein structure and function prediction". http://zhanglab.ccmb.med.umich.edu/I-TASSER/.
- ↑ BPS : A. W. Burgess and P. K. Ponnuswamy and H. A. Sheraga, Analysis of conformations of amino acid residues and prediction of backbone topography in proteins, Israel J. Chem., p239-286, 1974, vol12. D_R : G. Dele`age and B. Roux, An algorithm for secondary structure prediction based on class prediction, Protein Engineering, p289-294, 1987, vol 1, num 4. DSC : Ross D. King and Michael J.E. Sternberg - Identification and application of the concepts important for accurate and reliable protein secondary structure prediction. Protein Science, 1996, 5:2298-2310 GGR : Garnier, Gibrat, and Robson, Meth. Enzymol., R.F. Doolittle ed. 1996, 266:97-120 GOR : Jean Garnier and D. J. Osguthorpe and Barry Robson, Analysis of the accuracy and implications of simple methods for predicting the secondary structure of proteins, J. Mol. Biol., p 97-120, 1978, vol 120. G_G : O. Gascuel and J. L. Golmard, A simple method for predicting the secondary structure of globular proteins: implications and accuracy, CABIOS, p 357-365, 1988, vol 4. H_K : L. Howard Holley and Martin Karplus, Protein secondary structure prediction with a neural network, Proc. Natl. Acad. Sci. USA, p 152-156, Jan 1989, vol 86. K_S : Ross D. King and Michael J. E. Sternberg, Machine learning approach for the prediction of protein secondary structure, J. Mol. Biol., p 441-457, 1990, vol 216. L_G : Jonathan M. Levin and Jean Garnier, Improvements in a secondary structure prediction method based on a search for local sequence homologies and its use as a model building tool, Biochim. Biophys. Acta., p 283-295, 1988, vol 955. Q_S : Ning Qian and Terence Sejnowski, Predicting the secondary structure of proteins using neural network models, J. Mol. Biol., p 865-884, 1988, vol 202. JOI Joint prediction - Prediction made by the program that assigns the structure using a "winner takes all" procedure for each amino acid prediction using the other methods.
- ↑ "NetPhos 3.1 Server" (in en). http://www.cbs.dtu.dk/services/NetPhos/.
- ↑ "Motif Scan" (in en). http://myhits.isb-sib.ch/cgi-bin/motif_scan.
- ↑ "WikiGenes - Collaborative Publishing". https://www.wikigenes.org/e/gene/e/79401.html.
- ↑ 17.0 17.1 17.2 M. Zuker. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31 (13), 3406-15, (2003)
- ↑ "TargetScanHuman 7.1". http://www.targetscan.org/vert_71/.
- ↑ Desai A, Glaser A, Liu D, Raghavachari N et al. Microarray-based characterization of a colony assay used to investigate endothelial progenitor cells and relevance to endothelial function in humans. Arterioscler Thromb Vasc Biol 2009 Jan;29(1):121-7. PMID 19092138
- ↑ "TEX36 protein (Homo sapiens) - STRING network view". http://string-db.org/cgi/network.pl?taskId=PbbRE2pQzp4E.
- ↑ 21.0 21.1 BioGRID3.4 Result Summary on TEX36, [https://thebiogrid.org/132412]
- ↑ Fardilha, M., Esteves, S. L., Korrodi-Gregório, L., Vintém, A. P., Domingues, S. C., Rebelo, S., ... & e Silva, E. F. D. C. (2011). Identification of the human testis protein phosphatase 1 interactome. Biochemical Pharmacology, 82(10), 1403-1415.
- ↑ Yan, J., Hitomi, T., Takenaka, K., Kato, M., Kobayashi, H., Okuda, H., ... & Koizumi, A. (2015). Genetic study of intracranial aneurysms. Stroke, STROKEAHA-114.
- ↑ Lokman, F. E., Seman, N. A., al-Safi Ismail, A., Azwany Yaacob, N., Mustafa, N., Khir, A. S., ... & Wan Mohamud, W. N. (2011). Gene expression profiling in ethnic Malays with type 2 diabetes mellitus, with and without diabetic nephropathy. Journal of nephrology, 24(6), 778.
- ↑ Hitchcock, E., & Gibson, W. T. (2016). A Review of the Genetics of Intracranial Berry Aneurysms and Implications for Genetic Counseling. Journal of Genetic Counseling, 1-11.
- ↑ 26.0 26.1 "Human BLAT Search". https://genome.ucsc.edu/cgi-bin/hgBlat?command=start.
- ↑ PHYLIP: Felsenstein, J. 1993. PHYLIP (Phylogeny Inference Package) version 3.5c. Distributed by the author. Department of Genetics, University of Washington, Seattle.
- ↑ "Protein BLAST: search protein databases using a protein query" (in en). https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins.
- ↑ 29.0 29.1 Stephen F. Altschul, Thomas L. Madden, Alejandro A. Schäffer, Jinghui Zhang, Zheng Zhang, Webb Miller, and David J. Lipman (1997), "Gapped BLAST and PSI-BLAST: a new generation of protein database search programs", Nucleic Acids Res. 25:3389-3402.
- ↑ "TimeTree :: The Timescale of Life". http://www.timetree.org/.
 |

