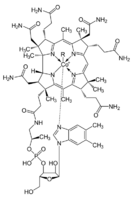
Biology:Cobalt in biology

Cobalt is essential to the metabolism of all animals. It is a key constituent of cobalamin, also known as vitamin B12, the primary biological reservoir of cobalt as an ultratrace element.[1][2] Bacteria in the stomachs of ruminant animals convert cobalt salts into vitamin B12, a compound which can only be produced by bacteria or archaea. A minimal presence of cobalt in soils therefore markedly improves the health of grazing animals, and an uptake of 0.20 mg/kg a day is recommended because they have no other source of vitamin B12.[3]
Proteins based on cobalamin use corrin to hold the cobalt. Coenzyme B12 features a reactive C-Co bond that participates in the reactions.[4] In humans, B12 has two types of alkyl ligand: methyl and adenosyl. MeB12 promotes methyl (−CH3) group transfers. The adenosyl version of B12 catalyzes rearrangements in which a hydrogen atom is directly transferred between two adjacent atoms with concomitant exchange of the second substituent, X, which may be a carbon atom with substituents, an oxygen atom of an alcohol, or an amine. Methylmalonyl coenzyme A mutase (MUT) converts MMl-CoA to Su-CoA, an important step in the extraction of energy from proteins and fats.[5]
Although far less common than other metalloproteins (e.g. those of zinc and iron), other cobaltoproteins are known besides B12. These proteins include methionine aminopeptidase 2, an enzyme that occurs in humans and other mammals that does not use the corrin ring of B12, but binds cobalt directly. Another non-corrin cobalt enzyme is nitrile hydratase, an enzyme in bacteria that metabolizes nitriles.[6]
Cobalt deficiency
In humans, consumption of cobalt-containing vitamin B12 meets all needs for cobalt. For cattle and sheep, which meet vitamin B12 needs via synthesis by resident bacteria in the rumen, there is a function for inorganic cobalt. In the early 20th century, during the development of farming on the North Island Volcanic Plateau of New Zealand, cattle suffered from what was termed "bush sickness". It was discovered that the volcanic soils lacked the cobalt salts essential for the cattle food chain.[7][8] The "coast disease" of sheep in the Ninety Mile Desert of the Southeast of South Australia in the 1930s was found to originate in nutritional deficiencies of trace elements cobalt and copper. The cobalt deficiency was overcome by the development of "cobalt bullets", dense pellets of cobalt oxide mixed with clay given orally for lodging in the animal's rumen.[clarification needed][9][8][10]
References
- ↑ Yamada, Kazuhiro (2013). "Chapter 9. Cobalt: Its Role in Health and Disease". in Astrid Sigel. Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences. 13. Springer. pp. 295–320. doi:10.1007/978-94-007-7500-8_9.
- ↑ Cracan, Valentin; Banerjee, Ruma (2013). "Chapter 10 Cobalt and Corrinoid Transport and Biochemistry". in Banci, Lucia. Metallomics and the Cell. Metal Ions in Life Sciences. 12. Springer. pp. 333–374. doi:10.1007/978-94-007-5561-1_10. ISBN 978-94-007-5560-4. electronic-book ISBN:978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402.
- ↑ Schwarz, F. J.; Kirchgessner, M.; Stangl, G. I. (2000). "Cobalt requirement of beef cattle – feed intake and growth at different levels of cobalt supply". Journal of Animal Physiology and Animal Nutrition 83 (3): 121–131. doi:10.1046/j.1439-0396.2000.00258.x.
- ↑ Voet, Judith G.; Voet, Donald (1995). Biochemistry. New York: J. Wiley & Sons. p. 675. ISBN 0-471-58651-X. OCLC 31819701. https://archive.org/details/biochemistry00voet_0/page/675.
- ↑ Smith, David M.; Golding, Bernard T.; Radom, Leo (1999). "Understanding the Mechanism of B12-Dependent Methylmalonyl-CoA Mutase: Partial Proton Transfer in Action". Journal of the American Chemical Society 121 (40): 9388–9399. doi:10.1021/ja991649a.
- ↑ Kobayashi, Michihiko; Shimizu, Sakayu (1999). "Cobalt proteins". European Journal of Biochemistry 261 (1): 1–9. doi:10.1046/j.1432-1327.1999.00186.x. PMID 10103026.
- ↑ "Soils". Waikato University. http://sci.waikato.ac.nz/farm/content/soils.html#bush_sickness.
- ↑ 8.0 8.1 McDowell, Lee Russell (2008). Vitamins in Animal and Human Nutrition (2nd ed.). Hoboken: John Wiley & Sons. p. 525. ISBN 978-0-470-37668-3. https://books.google.com/books?id=UR9MnQ806LsC&pg=PA525.
- ↑ Australian Academy of Science > Deceased Fellows > Hedley Ralph Marston 1900–1965 Accessed 12 May 2013.
- ↑ Snook, Laurence C. (1962). "Cobalt: its use to control wasting disease". Journal of the Department of Agriculture, Western Australia. 4 3 (11): 844–852. https://researchlibrary.agric.wa.gov.au/journal_agriculture4/vol3/iss11/2.
 |