Biology:KIX domain
| KIX (CBP) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
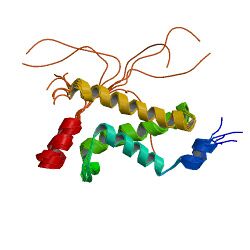 Complex between the KIX domain of CBP (yellow, green and cyan) and the C-terminal transactivation domain of p65/RELA (red)[1] | |||||||||
| Identifiers | |||||||||
| Symbol | KIX | ||||||||
| Pfam | PF02172 | ||||||||
| Pfam clan | CL0589 | ||||||||
| InterPro | IPR003101 | ||||||||
| CATH | 1sb0 | ||||||||
| SCOP2 | 1sb0 / SCOPe / SUPFAM | ||||||||
| |||||||||
| KIX (MED15) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | KIX_2 | ||||||||
| Pfam | PF16987 | ||||||||
| InterPro | IPR036546 | ||||||||
| |||||||||
In biochemistry, the KIX domain (kinase-inducible domain (KID) interacting domain) or CREB binding domain is a protein domain of the eukaryotic transcriptional coactivators CBP and P300. It serves as a docking site for the formation of heterodimers between the coactivator and specific transcription factors. Structurally, the KIX domain is a globular domain consisting of three α-helices and two short 310-helices.
The KIX domain was originally discovered in 1996 as the specific and minimal region in CBP that binds and interacts with phosphorylated CREB to activate transcription.[2] It was thus first termed CREB-binding domain. However, when it was later discovered that it also binds many other proteins, the more general name KIX domain became favoured. The KIX domain contains two separate binding sites: the "c-Myb site", named after the oncoprotein c-Myb, and the "MLL site", named after the proto-oncogene MLL (Mixed Lineage Leukemia, KMT2A).[3]
The paralogous coactivators CBP (CREBBP) and P300 (EP300) are recruited to DNA-bound transcription factors to activate transcription. Coactivators can associate with promoters and enhancers in the DNA only indirectly through protein-protein contacts with transcription factors. CBP and P300 activate transcription synergistically in two ways: first, by remodelling and relaxing chromatin through their intrinsic histone acetyltransferase activity, and second, by recruiting the basal transcription machinery, such as RNA polymerase II.[4]
The KIX domain belongs to the proposed GACKIX domain superfamily. GACKIX comprises structurally and functionally highly homologous domains in related proteins. It is named after the protein GAL11 / ARC105 (MED15), the plant protein CBP-like, and the KIX domain from CBP and P300.[5] Additional instances include RECQL5 and related plant proteins.[6][7] All of these contain a KIX domain or KIX-related domain that interacts with the transactivation domain of many different transcription factors. The distinction between a KIX domain, a KIX-related domain and a GACKIX domain is subject to an ongoing debate and not clearly defined.
The full CBP/P300 protein
Aside from the KIX domain, CBP and P300 contain many other protein binding domains that should not be confused (numbers are aa numberings):
- CH1/TAZ1 domain, CBP[347–433], P300[323-423][8]
- KIX domain, CBP[587–666], P300[566–645][8]
- Bromodomain, CBP[1103–1175], P300[1067–1139][8]
- CH2 domain (IPR010303), CBP[1191–1317], P300[1155-1280].[9]
- HAT domain, CBP[1323–1700], P300[1287–1663][8]
- CH3/ZZ domain, CBP[1701-1744], P300[1664-1707][8]
- CH3/TAZ2 domain, CBP[1765–1846], P300[1728-1809][8]
- IRF-3 binding (i-BiD),[10] nuclear receptor coactivator binding (NCBD),[11] or SRC1 interaction domain (SID; IPR014744),[12] CBP[2020-2113], P300[1992-2098].
All three CH (cysteine/histidine-rich) domains are zinc fingers.[9]
Interactions
Human and animal proteins:
Yeast proteins:
Viral proteins:
References
- ↑ 1.0 1.1 "Structural characterization of interactions between transactivation domain 1 of the p65 subunit of NF-κB and transcription regulatory factors". Nucleic Acids Research 45 (9): 5564–5576. May 2017. doi:10.1093/nar/gkx146. PMID 28334776.
- ↑ "Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism". Molecular and Cellular Biology 16 (2): 694–703. February 1996. doi:10.1128/MCB.16.2.694. PMID 8552098.
- ↑ "Cooperativity in transcription factor binding to the coactivator CREB-binding protein (CBP). The mixed lineage leukemia protein (MLL) activation domain binds to an allosteric site on the KIX domain". The Journal of Biological Chemistry 277 (45): 43168–74. November 2002. doi:10.1074/jbc.M207660200. PMID 12205094.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 "Molecular recognition by the KIX domain and its role in gene regulation". Nucleic Acids Research 42 (4): 2112–25. February 2014. doi:10.1093/nar/gkt1147. PMID 24253305.
- ↑ "Linking transcriptional mediators via the GACKIX domain super family". Current Biology 14 (2): R54-5. January 2004. doi:10.1016/j.cub.2003.12.042. PMID 14738747.
- ↑ "Recruitment and retention dynamics of RECQL5 at DNA double strand break sites". DNA Repair 11 (7): 624–35. July 2012. doi:10.1016/j.dnarep.2012.05.001. PMID 22633600.
- ↑ "KIXBASE: A comprehensive web resource for identification and exploration of KIX domains". Scientific Reports 7 (1): 14924. November 2017. doi:10.1038/s41598-017-14617-0. PMID 29097748. Bibcode: 2017NatSR...714924Y.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 UniProt and InterPro entry for CBP (Q92793) and P300 (Q09472)
- ↑ 9.0 9.1 "The CH2 domain of CBP/p300 is a novel zinc finger". FEBS Letters 587 (16): 2506–11. August 2013. doi:10.1016/j.febslet.2013.06.051. PMID 23831576.
- ↑ 10.0 10.1 "A small domain of CBP/p300 binds diverse proteins: solution structure and functional studies". Molecular Cell 8 (3): 581–90. September 2001. doi:10.1016/S1097-2765(01)00333-1. PMID 11583620.
- ↑ "Packing, specificity, and mutability at the binding interface between the p160 coactivator and CREB-binding protein". Protein Science 13 (1): 203–10. January 2004. doi:10.1110/ps.03366504. PMID 14691235.
- ↑ "Analysis of the steroid receptor coactivator 1 (SRC1)-CREB binding protein interaction interface and its importance for the function of SRC1". Molecular and Cellular Biology 21 (1): 39–50. January 2001. doi:10.1128/MCB.21.1.39-50.2001. PMID 11113179.
- ↑ "Transactivation mechanisms of mouse clock transcription factors, mClock and mArnt3". Genes to Cells 5 (9): 739–47. September 2000. doi:10.1046/j.1365-2443.2000.00363.x. PMID 10971655.
- ↑ "Cryptochrome 1 regulates the circadian clock through dynamic interactions with the BMAL1 C terminus". Nature Structural & Molecular Biology 22 (6): 476–484. June 2015. doi:10.1038/nsmb.3018. PMID 25961797.
- ↑ 15.00 15.01 15.02 15.03 15.04 15.05 15.06 15.07 15.08 15.09 15.10 15.11 15.12 "CREB-binding protein and p300 in transcriptional regulation". The Journal of Biological Chemistry 276 (17): 13505–8. April 2001. doi:10.1074/jbc.R000025200. PMID 11279224.
- ↑ "Synergistic interplay between promoter recognition and CBP/p300 coactivator recruitment by FOXO3a". ACS Chemical Biology 4 (12): 1017–27. December 2009. doi:10.1021/cb900190u. PMID 19821614.
- ↑ "Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3". The Journal of Biological Chemistry 274 (12): 8143–52. March 1999. doi:10.1074/jbc.274.12.8143. PMID 10075717.
- ↑ "MLL and CREB bind cooperatively to the nuclear coactivator CREB-binding protein". Molecular and Cellular Biology 21 (7): 2249–58. April 2001. doi:10.1128/MCB.21.7.2249-2258.2001. PMID 11259575.
- ↑ "CREB-binding protein/p300 are transcriptional coactivators of p65". Proceedings of the National Academy of Sciences of the United States of America 94 (7): 2927–32. April 1997. doi:10.1073/pnas.94.7.2927. PMID 9096323. Bibcode: 1997PNAS...94.2927G.
- ↑ "Regulation of E2A activities by histone acetyltransferases in B lymphocyte development". The Journal of Biological Chemistry 278 (4): 2370–6. January 2003. doi:10.1074/jbc.M211464200. PMID 12435739.
External links
- KIXBASE, a database of KIX-superfamily domains and PDB structures (HMM training dataset)
 |


