Biology:Stegodon
| Stegodon | |
|---|---|

| |
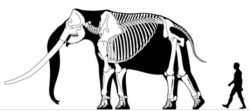
| Stegodon skeleton at the Gansu Provincial Museum | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Proboscidea |
| Family: | †Stegodontidae |
| Genus: | †Stegodon Falconer, 1847 |
| Species | |
| |
Stegodon ("roofed tooth" from the Ancient Greek words στέγω, stégō, 'to cover', + ὀδούς, odoús, 'tooth' because of the distinctive ridges on the animal's molars) is an extinct genus of proboscidean, related to elephants. It was originally assigned to the family Elephantidae along with modern elephants but is now placed in the extinct family Stegodontidae. Like elephants, Stegodon had teeth with plate-like lophs that are different from those of more primitive proboscideans like gomphotheres and mammutids.[1] The oldest fossils of the genus are found in Late Miocene strata in Asia, likely originating from the more archaic Stegolophodon, shortly afterwards migrating into Africa.[2] While the genus became extinct in Africa during the Pliocene, Stegodon remained widespread in South, Southeast and East Asia until the end of the Pleistocene.[3]
Morphology
The skull of Stegodon is relatively tall but short.[1] The lower jaw in comparison to early elephantimorphs is shortened (brevirostrine), and lacks lower tusks/incisors. The molar teeth are superficially like those of elephants, consisting of parallel lamellae that form ridges but are generally relatively low crowned (brachydont),[1][4] the numbers of ridges are greater in later species.[5] Members of the genus lack permanent premolars.[6] The tusks are proportionally large, with those of the biggest species being among the largest known tusks in proboscideans, with a particularly large tusk of S. ganesa from the Early Pleistocene of India measured to be 3.89 metres (12.8 ft) long, with an estimated mass of approximately 140 kilograms (310 lb), substantially larger than the largest recorded modern elephant tusk.[7]
Size
Some species of Stegodon were amongst the largest proboscideans. The Chinese species S. zdanskyi is known from an old male (50-plus years old) from the Yellow River that is 3.87 m (12.7 ft) tall and would have weighed approximately 12.7 tonnes (12.5 long tons; 14.0 short tons) in life. It had a humerus 1.21 m (4.0 ft) long, a femur 1.46 m (4.8 ft) long, and a pelvis 2 m (6.6 ft) wide. The Indian S. ganesa is suggested to have a shoulder height of about 3.10 m (10.2 ft), and a body mass of around 6.5 tonnes (6.4 long tons; 7.2 short tons). The Javanese species S. trigonocephalus is suggested to have been around 2.75–2.8 m (9.0–9.2 ft) tall, with a body mass of around 5 tonnes (4.9 long tons; 5.5 short tons).[8]
Dwarfism
Similar to modern-day elephants, stegodonts were likely good swimmers,[9][10] allowing them to disperse to remote islands in Indonesia, the Philippines and Japan. Once present on the islands, due to the process of insular dwarfism, as a result of decreased land area and the reduction of predation and competition pressure, they reduced in body size, with the degree of dwarfism varying between islands as the result of local conditions. One of the smallest species, Stegodon sumbaensis from Sumba in Indonesia, is estimated at around 8% of the size of mainland Stegodon species, with a body mass of 250 kilograms (550 lb).[11] Sometimes the same island was colonised multiple times by Stegodon, as in Flores, where the Early Pleistocene strongly dwarfed species Stegodon sondaari, which was 120 cm (3.9 ft) tall at the shoulder and weighed about 350–400 kilograms (770–880 lb),[8] was replaced by the species Stegodon florensis during the Middle Pleistocene which was initially substantially larger, but progressively reduced in size over time, with the earlier subspecies Stegodon florensis florensis from the Middle Pleistocene estimated to be around 50% the size of mainland Stegodon species with a shoulder height of around 190 cm (6.2 ft) and a body mass of around 1.7 tons, while the later Stegodon florensis insularis from the Late Pleistocene is estimated to be around 17% the size of mainland Stegodon species, with a shoulder height of around 130 cm (4.3 ft), and a body mass of about 570 kilograms (1,260 lb).[11][12]
Ecology
Like modern elephants, but unlike more primitive proboscideans, Stegodon is thought to have chewed using a proal movement (a forward stroke from the back to the front) of the lower jaws. This jaw movement is thought to have evolved independently in elephants and stegodontids.[1] Stegodon populations from the Pliocene of the India are suggested to have been variable mixed feeders based on isotopic analysis.[13] Based on dental microwear analysis, populations of Stegodon from the Pleistocene of China (Stegodon orientalis and Stegodon huananensis) and mainland southeast Asia (S. orientalis) were found to be browsers, with clear niche differentiation from sympatric Elephas populations, which tended towards mixed feeding (both browsing and grazing).[14][15] In contrast, specimens of Stegodon trigonocephalus from the Early-Middle Pleistocene of Java were found to be mixed feeders to grazers, with a diet similar to that of sympatric Elephas hysudrindicus.[16] The dwarf species from Flores, Stegodon sondaari and Stegodon florensis, are suggested to have been mixed feeders and grazers, respectively, based on stable carbon isotopes.[12] Specimens of Stegodon kaiesensis from the Pliocene of East Africa were found to be browsers to mixed feeders, based on mesowear analysis.[17]
Tracks of a group of Stegodon from the Late Pliocene of Japan suggest that like modern elephants, Stegodon lived in social herds.[18]
On Flores, where dwarf Stegodon species were the only large herbivores, they were likely the main prey of the Komodo dragon.[19] Claims that Stegodon florensis was hunted by Homo floresiensis are based on ambiguous circumstantial association between bones and stone tools, and the rarity of cut marks makes it unclear to what if to any degree, hunting of Stegodon was actually practiced by Homo floresiensis.[20][21]
Taxonomy
In the past, stegodonts were believed to be the ancestors of the true elephants and mammoths, but currently they are believed to have no modern descendants. Stegodon is likely derived from Stegolophodon, an extinct genus known from the Miocene of Asia,[2] with transitional fossils between the two genera known from the Late Miocene of Southeast Asia and Yunnan in South China.[1] Stegodon is more closely related to elephants and mammoths than to mastodons.[22] Like elephants, stegodontids are believed to have derived from gomphotheres.[23]
Phylogeny
The following cladogram shows the placement of the genus Stegodon among other proboscideans, based on hyoid characteristics:[22]
| ||||||||||||||||||||||||||||||||||
List of species
- Stegodon kaisensis Late Miocene – Pliocene, Africa
- Stegodon zdanskyi Late Miocene – Pliocene, China
- Stegodon huananensis Early Pleistocene, China
- Stegodon orientalis Middle – Late Pleistocene, China, Southeast Asia, Japan, Taiwan
- Stegodon namadicus/S. insignis/S. ganesa Pliocene – Late Pleistocene, India
- Stegodon miensis Pliocene, Japan
- Stegodon protoaurorae Late Pliocene – Early Pleistocene, Japan
- Stegodon aurorae Early Pleistocene – early Middle Pleistocene, Japan
- Stegodon sondaari Early Pleistocene, Flores, Indonesia
- Stegodon florensis Middle – Late Pleistocene, Flores, Indonesia
- Stegodon luzonensis Middle Pleistocene, Luzon, Philippines
- Stegodon trigonocephalus late Early Pleistocene – early Late Pleistocene, Java, Indonesia
- Stegodon sompoensis Late Pliocene – Early Pleistocene, Sulawesi, Indonesia
- Stegodon sumbaensis Middle – Late Pleistocene, Sumba, Indonesia
- Stegodon timorensis Middle Pleistocene, Timor, Indonesia
- Stegodon mindanensis Pleistocene Mindanao, Philippines
An indeterminate Stegodon molar of an uncertain locality and age is known from Greece, representing the only record of the genus in Europe.[24] Indeterminate remains are also known from the Early Pleistocene and early Middle Pleistocene of Israel.[25]
Evolution and extinction
The oldest fossils of Stegodon in Asia date to the Late Miocene, around 8-11 million years ago,[2] with the oldest fossils of the genus in Africa being around 7-6 million years old.[26] Stegodon became extinct in Africa during the late Pliocene, around 3 million years ago suggested to be the result of expansion of grassland habitats.[17] The Javanese species Stegodon trigonocephalus became extinct around 130-80,000 years ago during the latest Middle Pleistocene-early Late Pleistocene (Marine Isotope Stage 5) following a change to more humid conditions, which may have reduced grazing habitat.[16] Stegodon florensis became extinct around 50,000 years ago, around the time of arrival of modern humans to Flores.[27] Stegodon became extinct in the Indian subcontinent (Stegodon namadicus/Stegodon sp.), mainland Southeast Asia and China (S. orientalis) at some point during the Late Pleistocene epoch, while Asian elephants, which existed in sympatry with Stegodon in these regions, are still extant. The precise timing of extinction is uncertain for these regions.[28][15][29] The survival of the Asian elephant as opposed to Stegodon orientalis in Southeast Asia and South China has been suggested to be due to its more flexible diet in comparison to S. orientalis.[15] A review of 130 papers written about 180 different sites with proboscidean remains in southern China revealed Stegodon to have been more common than Asian elephants; the papers gave many recent radiocarbon dates, the youngest being 2,150 BCE (4,100 BP).[30] However, Turvey et al. (2013) reported that one of the faunal assemblages including supposed fossils of Holocene Stegodon (from Gulin, Sichuan Province) is actually late Pleistocene in age; other supposed fossils of Holocene stegodonts were lost and their age cannot be verified. The authors concluded that the latest confirmed occurrences of Stegodon from China are from the Late Pleistocene, and that its Holocene survival cannot be substantiated.[29]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Saegusa, Haruo (March 2020). "Stegodontidae and Anancus: Keys to understanding dental evolution in Elephantidae" (in en). Quaternary Science Reviews 231: 106176. doi:10.1016/j.quascirev.2020.106176. Bibcode: 2020QSRv..23106176S. https://linkinghub.elsevier.com/retrieve/pii/S0277379119302665.
- ↑ 2.0 2.1 2.2 Ao, Hong; Zhang, Peng; Dekkers, Mark J.; Roberts, Andrew P.; An, Zhisheng; Li, Yongxiang; Lu, Fengyan; Lin, Shan et al. (January 2016). "New magnetochronology of Late Miocene mammal fauna, NE Tibetan Plateau, China: Mammal migration and paleoenvironments" (in en). Earth and Planetary Science Letters 434: 220–230. doi:10.1016/j.epsl.2015.11.019. Bibcode: 2016E&PSL.434..220A. https://linkinghub.elsevier.com/retrieve/pii/S0012821X15007232.
- ↑ O’Regan, H. J.; Bishop, L. C.; Lamb, A.; Elton, S.; Turner, A. (2005-01-01). "Large mammal turnover in Africa and the Levant between 1.0 and 0.5 Ma" (in en). Geological Society, London, Special Publications 247 (1): 231–249. doi:10.1144/GSL.SP.2005.247.01.13. ISSN 0305-8719. Bibcode: 2005GSLSP.247..231O. https://sp.lyellcollection.org/content/247/1/231.
- ↑ Cantalapiedra, Juan L.; Sanisidro, Óscar; Zhang, Hanwen; Alberdi, María T.; Prado, José L.; Blanco, Fernando; Saarinen, Juha (2021-07-01). "The rise and fall of proboscidean ecological diversity" (in en). Nature Ecology & Evolution 5 (9): 1266–1272. doi:10.1038/s41559-021-01498-w. ISSN 2397-334X. PMID 34211141. Bibcode: 2021NatEE...5.1266C. https://www.nature.com/articles/s41559-021-01498-w.
- ↑ van der Made, J. The Evolution of the Elephants and Their Relatives in the Context of Changing Climate and Geography. In Elefantentreich—Eine Fossilwelt in Europa; Verlag Beier & Beran: Langenweißbach, Germany, 2010; pp. 340–360. ISBN 978-3-939414-48-3.
- ↑ Sanders, William J. (2018-02-17). "Horizontal tooth displacement and premolar occurrence in elephants and other elephantiform proboscideans" (in en). Historical Biology 30 (1–2): 137–156. doi:10.1080/08912963.2017.1297436. ISSN 0891-2963. Bibcode: 2018HBio...30..137S. https://www.tandfonline.com/doi/full/10.1080/08912963.2017.1297436.
- ↑ Larramendi, Asier (2023-12-10). "Estimating tusk masses in proboscideans: a comprehensive analysis and predictive model" (in en). Historical Biology: 1–14. doi:10.1080/08912963.2023.2286272. ISSN 0891-2963. https://www.tandfonline.com/doi/full/10.1080/08912963.2023.2286272.
- ↑ 8.0 8.1 Larramendi, A. (2016). "Shoulder height, body mass and shape of proboscideans". Acta Palaeontologica Polonica 61. doi:10.4202/app.00136.2014. https://www.app.pan.pl/archive/published/app61/app001362014.pdf.
- ↑ Simpson, G. (1977). "Too Many Lines; The Limits of the Oriental and Australian Zoogeographic Regions". Proceedings of the American Philosophical Society, 121(2), 107–120. Retrieved from http://www.jstor.org/stable/986523
- ↑ Bird, Michael I.; Condie, Scott A.; O’Connor, Sue; O’Grady, Damien; Reepmeyer, Christian; Ulm, Sean; Zega, Mojca; Saltré, Frédérik et al. (2019). "Early human settlement of Sahul was not an accident". Scientific Reports 9 (1): 8220. doi:10.1038/s41598-019-42946-9. PMID 31209234. Bibcode: 2019NatSR...9.8220B.
- ↑ 11.0 11.1 Geer, Alexandra A. E.; Bergh, Gerrit D.; Lyras, George A.; Prasetyo, Unggul W.; Due, Rokus Awe; Setiyabudi, Erick; Drinia, Hara (August 2016). "The effect of area and isolation on insular dwarf proboscideans" (in en). Journal of Biogeography 43 (8): 1656–1666. doi:10.1111/jbi.12743. ISSN 0305-0270. Bibcode: 2016JBiog..43.1656V. https://onlinelibrary.wiley.com/doi/10.1111/jbi.12743.
- ↑ 12.0 12.1 Puspaningrum, Mika; Van Den Bergh, Gerrit; Chivas, Allan; Setiabudi, Erick; Kurniawan, Iwan; Brumm, Adam; and Sutikna, Thomas, "Preliminary results of dietary and environmental reconstructions of Early to Middle Pleistocene Stegodons from the So'a Basin of Flores, Indonesia, based on enamel stable isotope records" (2014). Faculty of Science, Medicine and Health - Papers: part A. 2035.
- ↑ Patnaik, Rajeev; Singh, Ningthoujam Premjit; Paul, Debajyoti; Sukumar, Raman (November 2019). "Dietary and habitat shifts in relation to climate of Neogene-Quaternary proboscideans and associated mammals of the Indian subcontinent" (in en). Quaternary Science Reviews 224: 105968. doi:10.1016/j.quascirev.2019.105968. Bibcode: 2019QSRv..22405968P. https://linkinghub.elsevier.com/retrieve/pii/S027737911930263X.
- ↑ Zhang, Hanwen; Wang, Yuan; Janis, Christine M.; Goodall, Robert H.; Purnell, Mark A. (2017-07-25). "An examination of feeding ecology in Pleistocene proboscideans from southern China (Sinomastodon, Stegodon, Elephas), by means of dental microwear texture analysis" (in en). Quaternary International. VIth International Conference on Mammoths and their Relatives, Part 3 445: 60–70. doi:10.1016/j.quaint.2016.07.011. ISSN 1040-6182. Bibcode: 2017QuInt.445...60Z.
- ↑ 15.0 15.1 15.2 Ma, Jiao; Wang, Yuan; Jin, Changzhu; Hu, Yaowu; Bocherens, Hervé (2019-05-15). "Ecological flexibility and differential survival of Pleistocene Stegodon orientalis and Elephas maximus in mainland southeast Asia revealed by stable isotope (C, O) analysis" (in en). Quaternary Science Reviews 212: 33–44. doi:10.1016/j.quascirev.2019.03.021. ISSN 0277-3791. Bibcode: 2019QSRv..212...33M. https://www.sciencedirect.com/science/article/pii/S0277379118309648.
- ↑ 16.0 16.1 Puspaningrum, Mika R.; van den Bergh, Gerrit D.; Chivas, Allan R.; Setiabudi, Erick; Kurniawan, Iwan (January 2020). "Isotopic reconstruction of Proboscidean habitats and diets on Java since the Early Pleistocene: Implications for adaptation and extinction" (in en). Quaternary Science Reviews 228: 106007. doi:10.1016/j.quascirev.2019.106007. Bibcode: 2020QSRv..22806007P. https://linkinghub.elsevier.com/retrieve/pii/S0277379119303178.
- ↑ 17.0 17.1 Saarinen, Juha; Lister, Adrian M. (2023-08-14). "Fluctuating climate and dietary innovation drove ratcheted evolution of proboscidean dental traits" (in en). Nature Ecology & Evolution 7 (9): 1490–1502. doi:10.1038/s41559-023-02151-4. ISSN 2397-334X. Bibcode: 2023NatEE...7.1490S.
- ↑ Matsukawa, Masaki; Shibata, Kenichiro (2015-10-02). "Review of Japanese Cenozoic (Miocene–Modern) Vertebrate Tracks" (in en). Ichnos 22 (3–4): 261–290. doi:10.1080/10420940.2015.1064407. ISSN 1042-0940. Bibcode: 2015Ichno..22..261M. http://www.tandfonline.com/doi/full/10.1080/10420940.2015.1064407.
- ↑ Diamond, Jared M. (1987). "Did Komodo dragons evolve to eat pygmy elephants?". Nature. 326 (6116): 832.
- ↑ Matthew W. Tocheri, Thomas Sutikna, Jatmiko, E. Wahyu Saptomo (2022-02-14), "Homo floresiensis", The Oxford Handbook of Early Southeast Asia (Oxford University Press), doi:10.1093/oxfordhb/9780199355358.013.2, https://academic.oup.com/edited-volume/42054/chapter-abstract/355842802, retrieved 2023-03-13
- ↑ Sutikna, Thomas; Tocheri, Matthew W.; Faith, J. Tyler; Jatmiko; Due Awe, Rokus; Meijer, Hanneke J.M.; Wahyu Saptomo, E.; Roberts, Richard G. (November 2018). "The spatio-temporal distribution of archaeological and faunal finds at Liang Bua (Flores, Indonesia) in light of the revised chronology for Homo floresiensis" (in en). Journal of Human Evolution 124: 52–74. doi:10.1016/j.jhevol.2018.07.001. PMID 30173885.
- ↑ 22.0 22.1 Shoshani, J.; Tassy, P. (2005). "Advances in proboscidean taxonomy & classification, anatomy & physiology, and ecology & behavior". Quaternary International 126–128: 5–20. doi:10.1016/j.quaint.2004.04.011. Bibcode: 2005QuInt.126....5S.
- ↑ Wu, Yan; Deng, Tao; Hu, Yaowu; Ma, Jiao; Zhou, Xinying; Mao, Limi; Zhang, Hanwen; Ye, Jie et al. (2018-05-16). "A grazing Gomphotherium in Middle Miocene Central Asia, 10 million years prior to the origin of the Elephantidae" (in en). Scientific Reports 8 (1): 7640. doi:10.1038/s41598-018-25909-4. ISSN 2045-2322. PMID 29769581. Bibcode: 2018NatSR...8.7640W.
- ↑ Konidaris, George E.; Tsoukala, Evangelia (2022), Vlachos, Evangelos, ed., "The Fossil Record of the Neogene Proboscidea (Mammalia) in Greece" (in en), Fossil Vertebrates of Greece Vol. 1 (Cham: Springer International Publishing): pp. 299–344, doi:10.1007/978-3-030-68398-6_12, ISBN 978-3-030-68397-9, https://link.springer.com/10.1007/978-3-030-68398-6_12, retrieved 2023-02-28
- ↑ Lister, Adrian M.; Dirks, Wendy; Assaf, Amnon; Chazan, Michael; Goldberg, Paul; Applbaum, Yaakov H.; Greenbaum, Nathalie; Horwitz, Liora Kolska (September 2013). "New fossil remains of Elephas from the southern Levant: Implications for the evolutionary history of the Asian elephant" (in en). Palaeogeography, Palaeoclimatology, Palaeoecology 386: 119–130. doi:10.1016/j.palaeo.2013.05.013. https://linkinghub.elsevier.com/retrieve/pii/S003101821300237X.
- ↑ Saegusa, Haruo; Thasod, Yupa; Ratanasthien, Benjavun (2005). "Notes on Asian stegodontids" (in en). Quaternary International 126-128: 31–48. doi:10.1016/j.quaint.2004.04.013. Bibcode: 2005QuInt.126...31S. https://linkinghub.elsevier.com/retrieve/pii/S1040618204000758.
- ↑ van den Bergh, Gerrit D.; Alloway, Brent V.; Storey, Michael; Setiawan, Ruly; Yurnaldi, Dida; Kurniawan, Iwan; Moore, Mark W.; Jatmiko et al. (October 2022). "An integrative geochronological framework for the Pleistocene So'a basin (Flores, Indonesia), and its implications for faunal turnover and hominin arrival" (in en). Quaternary Science Reviews 294: 107721. doi:10.1016/j.quascirev.2022.107721. Bibcode: 2022QSRv..29407721V. https://linkinghub.elsevier.com/retrieve/pii/S0277379122003523.
- ↑ Jukar, A. M.; Lyons, S. K.; Wagner, P. J.; Uhen, M. D. (2021-01-15). "Late Quaternary extinctions in the Indian Subcontinent" (in en). Palaeogeography, Palaeoclimatology, Palaeoecology 562: 110137. doi:10.1016/j.palaeo.2020.110137. ISSN 0031-0182. Bibcode: 2021PPP...56210137J.
- ↑ 29.0 29.1 Samuel T. Turvey, Haowen Tong, Anthony J. Stuart and Adrian M. Lister (2013). "Holocene survival of Late Pleistocene megafauna in China: a critical review of the evidence". Quaternary Science Reviews 76: 156–166. doi:10.1016/j.quascirev.2013.06.030. Bibcode: 2013QSRv...76..156T.
- ↑ H. Saegusa, "Comparisons of Stegodon and Elephantid Abundances in the Late Pleistocene of Southern China" , The World of Elephants – Second International Congress, (Rome, 2001), 345–349.
Wikidata ☰ Q772364 entry
 |