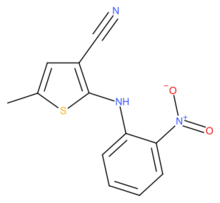
Chemistry:5-Methyl-2-((2-nitrophenyl)amino)-3-thiophenecarbonitrile
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-Methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile | |
| Other names
ROY
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties[1] | |
| C12H9N3O2S | |
| Molar mass | 259.28 g·mol−1 |
| Melting point | 99–102 °C (210–216 °F; 372–375 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H410 | |
| P273, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
5-Methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile, also known as ROY (red-orange-yellow), is an organic compound which is a chemical intermediate to the drug olanzapine. It has been the subject of intensive study because it can exist in multiple well-characterised crystalline polymorphic forms.[3][4][5][6]
Synthesis
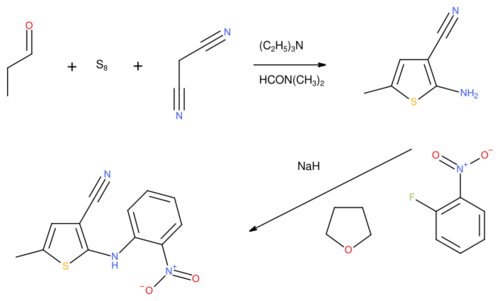
The preparation of ROY was first disclosed in a series of patents from Eli Lilly & Co. in the 1990s, which covered the pharmaceutical active ingredient later marketed as olanzapine. In the first step, a Gewald reaction using propionaldehyde, sulfur and malononitrile formed the thiophene ring system, as 2-amino-5-methylthiophene-3-carbonitrile. The amino group was then reacted with 2-fluoro-nitrobenzene in tetrahydrofuran to provide 5-methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile.[1]
Polymorphism
ROY has been crystallised in at least thirteen polymorphic forms.[7][8] Five of them, including red, orange and yellow examples are shown in Figure 1.
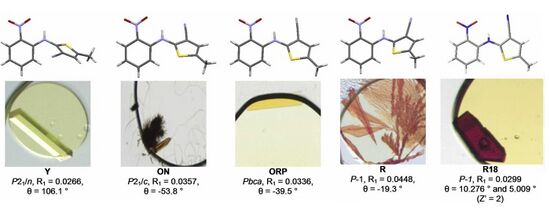
When this ability to form multiple crystalline versions of different colours was reviewed in 2010, it was described as "extraordinary", particularly because many alternatives can crystallise simultaneously from a single solvent. As the thermodynamic properties of the various versions have been established, ROY has become an important test of computational models.[4] By 2020, ROY held the record for having the largest number of well-characterised polymorphs, with its nearest competitors being aripiprazole and galunisertib.[9][10][11] The various crystal forms display alternative conformers, a type of stereoisomerism where rotation at single bonds leads to a distinct three-dimensional configuration in the solid.[12] The molecule is piezochromic, with yellow and pale orange crystalline forms which transform reversibly to red at high pressure.[8]
In 2022, it was suggested that all the ROY polymorphs which are stable at ambient pressure have already been found and characterised. This work also calculated that additional polymorphs might be discovered using high pressures of about 10 GPa.[11]
References
- ↑ 1.0 1.1 ; Hotten, T.M. & Tupper, D.E."Methods of treatment using a thieno-benzodiazepine" US patent 5817655, issued 1998-10-06, assigned to Eli Lilly and Co. Ltd.
- ↑ "5-Methyl-2-[(2-nitrophenyl)amino-3-thiophenecarbonitrile"]. https://pubchem.ncbi.nlm.nih.gov/compound/395460#section=GHS-Classification.
- ↑ Krämer, Katrina (2020-07-29). "Red–orange–yellow reclaims polymorph record with help from molecular cousin". https://www.chemistryworld.com/news/red-orange-yellow-reclaims-polymorph-record-with-help-from-molecular-cousin/4012160.article.
- ↑ 4.0 4.1 Yu, Lian (2010). "Polymorphism in Molecular Solids: An Extraordinary System of Red, Orange, and Yellow Crystals". Accounts of Chemical Research 43 (9): 1257–1266. doi:10.1021/ar100040r. PMID 20560545.
- ↑ Li, Xizhen; Ou, Xiao; Rong, Haowei; Huang, Siyong; Nyman, Jonas; Yu, Lian; Lu, Ming (2020). "The Twelfth Solved Structure of ROY: Single Crystals of Y04 Grown from Melt Microdroplets". Crystal Growth & Design 20 (11): 7093–7097. doi:10.1021/acs.cgd.0c01017.
- ↑ 6.0 6.1 Tyler, Andrew R.; Ragbirsingh, Ronnie; McMonagle, Charles J.; Waddell, Paul G.; Heaps, Sarah E.; Steed, Jonathan W.; Thaw, Paul; Hall, Michael J. et al. (2020). "Encapsulated Nanodroplet Crystallization of Organic-Soluble Small Molecules". Chem 6 (7): 1755–1765. doi:10.1016/j.chempr.2020.04.009. PMID 32685768.
- ↑ "5-methyl-2-(2-nitroanilino)thiophene-3-carbonitrile". https://www.ccdc.cam.ac.uk/structures/Search?Compound=5-methyl-2-(2-nitroanilino)thiophene-3-carbonitrile&DatabaseToSearch=Published.
- ↑ 8.0 8.1 Warren, Lisette R; McGowan, Evana; Renton, Margaret; Morrison, Carole A; Funnell, Nicholas P (2021). "Direct evidence for distinct colour origins in ROY polymorphs". Chemical Science 12 (38): 12711–12718. doi:10.1039/d1sc04051k. PMID 34703557.
- ↑ Reutzel-Edens, Susan M.; Bhardwaj, Rajni M. (2020). "Crystal forms in pharmaceutical applications: Olanzapine, a gift to crystal chemistry that keeps on giving". IUCrJ 7 (6): 955–964. doi:10.1107/S2052252520012683. PMID 33209310.
- ↑ Lévesque, Alexandre; Maris, Thierry; Wuest, James D. (2020). "ROY Reclaims Its Crown: New Ways to Increase Polymorphic Diversity". Journal of the American Chemical Society 142 (27): 11873–11883. doi:10.1021/jacs.0c04434. PMID 32510946.
- ↑ 11.0 11.1 Beran, Gregory J. O.; Sugden, Isaac J.; Greenwell, Chandler et al. (2022). "How many more polymorphs of ROY remain undiscovered". Chemical Science 13 (5): 1288–1297. doi:10.1039/d1sc06074k. PMID 35222912.
- ↑ Cruz-Cabeza, Aurora J.; Bernstein, Joel (2014). "Conformational Polymorphism". Chemical Reviews 114 (4): 2170–2191. doi:10.1021/cr400249d. PMID 24350653.
 |