Chemistry:Thiophene-2-carboxaldehyde
From HandWiki
Revision as of 02:47, 21 September 2021 by imported>Importwiki (fixing)
| |
| Names | |
|---|---|
| Preferred IUPAC name
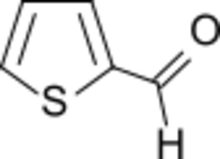
Thiophene-2-carbaldehyde | |
| Other names
2-formylthiophene, thiophene-2-aldehyde, T2A, 2-thiophenecarboxaldehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H4OS | |
| Molar mass | 112.15 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.2 g/mL |
| Boiling point | 198 °C (388 °F; 471 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H315, H317, H319, H335 | |
| P261, P264, P270, P271, P272, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P333+313, P337+313, P362, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thiophene-2-carboxaldehyde is an organosulfur compound with the formula C4H3SCHO. It is one of two isomeric thiophenecarboxaldehydes. It is a colorless liquid that often appears amber after storage. It is versatile precursor to many drugs including eprosartan, Azosemide, and Teniposide.
Preparation
It can be prepared from thiophene by the Vilsmeier reaction.[1] Alternatively, it is prepared from chloromethylation of thiophene.[2]
References
- ↑ Jonathan Swanston (2006). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_793.pub2.
- ↑ Kenneth B. Wiberg. "2-Thiophenealdehyde". Org. Synth. 3: 811. doi:10.15227/orgsyn.000.0005.
 |

