Chemistry:Teniposide
 | |
| Clinical data | |
|---|---|
| Trade names | Vumon |
| Other names | VM-26 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692045 |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | >99% |
| Metabolism | Hepatic (CYP2C19-mediated) |
| Elimination half-life | 5 hours |
| Excretion | Renal and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
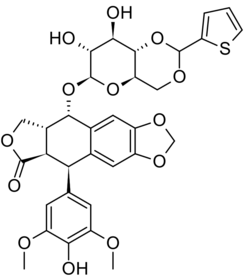
| Formula | C32H32O13S |
| Molar mass | 656.66 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Teniposide (trade name Vumon) is a chemotherapeutic medication[1] used in the treatment of childhood acute lymphocytic leukemia (ALL), Hodgkin's lymphoma, certain brain tumours, and other types of cancer.[2] It is in a class of drugs known as podophyllotoxin derivatives and slows the growth of cancer cells in the body.[3]
Medical uses
Teniposide is used for the treatment of a number of cancer types in children. In the US, it is approved for the second-line therapy of acute lymphocytic leukemia (ALL) in combination with other antineoplastic drugs.[3] In Europe, it is also approved for the treatment of Hodgkin's lymphoma, generalized malignant lymphoma, reticulocyte sarcoma, acute leukaemia, primary brain tumours (glioblastoma, ependymoma, astrocytoma), bladder cancer, neuroblastoma and other solid tumours in children.[2]
Administration
The medication is injected though a vein and burns if it leaks under the skin. It can be used in combination with other anticancer drugs.[2]
Contraindications
The drug is contraindicated during pregnancy and lactation, in patients with severe liver or kidney impairment or severely impaired haematopoiesis.[2]
Side effects
Teniposide, when used with other chemotherapeutic agents for the treatment of ALL, results in severe bone marrow suppression. Other common side effects include gastrointestinal toxicity, hypersensitivity reactions, and reversible alopecia.[2]
Interactions
No systematic interaction studies are available. The enzyme inducers phenobarbital and phenytoin have been found to lower its blood plasma concentrations.[4] Theoretically possible interactions include increased plasma concentrations when combined with sodium salicylate, sulfamethizole or tolbutamide, which displace teniposide from plasma protein binding, at least in vitro.[2][3]
Pharmacology
Mechanism of action
Teniposide causes dose-dependent single- and double-stranded breaks in DNA and DNA-protein cross-links.[2] The substance has been found to act as an inhibitor of topoisomerase II (an enzyme that aids in DNA unwinding),[4][5] since it does not intercalate into DNA or bind strongly to DNA. The cytotoxic effects of teniposide are related to the relative number of double-stranded DNA breaks produced in cells, which are a reflection of the stabilization of a topoisomerase II-DNA intermediate.[citation needed]
Chemistry
Teniposide is a semisynthetic derivative of podophyllotoxin[2] from the rhizome of the wild mandrake (Podophyllum peltatum). More specifically, it is a glycoside of podophyllotoxin with a D-glucose derivative. It is chemically similar to the anti-cancer drug etoposide, being distinguished only by a thienyl rest where etoposide has a methyl.[4] Both these compounds have been developed with the aim of creating less toxic derivatives of podophyllotoxin.[6]
References
- ↑ "Plants as a source of anti-cancer agents". Journal of Ethnopharmacology 100 (1–2): 72–79. August 2005. doi:10.1016/j.jep.2005.05.011. PMID 16009521. https://zenodo.org/record/1259111.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 (in German) Austria-Codex (62nd ed.). Vienna: Österreichischer Apothekerverlag. 2007. pp. 8855–6. ISBN 978-3-85200-181-4.
- ↑ 3.0 3.1 3.2 Drugs.com: Teniposide Monograph.
- ↑ 4.0 4.1 4.2 (in German) Arzneimittelwirkungen (8th ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. 2001. pp. 894–5. ISBN 3-8047-1763-2.
- ↑ "Topoisomerase II as a target of VM-26 and 4'-(9-acridinylamino)methanesulfon-m-aniside in atypical multidrug resistant human small cell lung carcinoma cells". Cancer Research 53 (5): 1064–1071. March 1993. PMID 8382551.
- ↑ (in German) Arzneistoff-Profile. 4 (28th ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. 2015. ISBN 978-3-7741-9846-3.
 |
