Chemistry:2-Hexanone
From HandWiki
Revision as of 01:02, 3 July 2022 by imported>Scavis2 (fix)
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexan-2-one | |
| Other names
Methyl butyl ketone; Methyl n-butyl ketone; MNBK; Butyl methyl ketone; MBK; n-Butyl methyl ketone; Propylacetone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
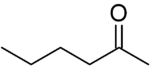
| C6H12O | |
| Molar mass | 100.161 g·mol−1 |
| Appearance | Colorless to light yellow liquid |
| Odor | sharp, acetone-like[3] |
| Density | 0.8113 g/cm3 |
| Melting point | −55.5 °C (−67.9 °F; 217.7 K) |
| Boiling point | 127.6 °C (261.7 °F; 400.8 K) |
| 1.4% (14 g/L) | |
| Vapor pressure | 1.3 kPa (20 °C) |
| -69.1·10−6 cm3/mol | |
Refractive index (nD)
|
1.403 (20 °C) |
| Viscosity | 0.63 mPa·s (20 °C) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H226, H336, H361f, H372 | |
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P281, P303+361+353, P304+340, P308+313, P312, P314, P370+378, P403+233, P403+235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 25 °C (77 °F; 298 K) |
| 423 °C (793 °F; 696 K) | |
| Explosive limits | ?-8%[3] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2590 mg/kg (oral, rat) 2430 mg/kg (oral, mouse) 4860 mg/kg (dermal, rabbit) 2590 mg/kg (oral, guinea pig)[4] |
LDLo (lowest published)
|
914 mg/kg (rat, oral)[4] |
LC50 (median concentration)
|
8000 ppm (rat, 4 hr)[4] |
LCLo (lowest published)
|
20,000 ppm (guinea pig, 70 min)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 100 ppm (410 mg/m3)[3] |
REL (Recommended)
|
TWA 1 ppm (4 mg/m3)[3] |
IDLH (Immediate danger)
|
1600 ppm[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2-Hexanone (methyl butyl ketone, MBK) is a ketone used as a general solvent and in paints. It dissolves cellulose nitrate, vinyl polymers and copolymers, and natural and synthetic resins. It is recommended as a solvent because it is photochemically inactive;[5] however it has a very low safe threshold limit value. 2-Hexanone is absorbed through the lungs, orally and dermally and its metabolite, 2,5-hexanedione, is neurotoxic.[6] Animal tests have shown that the neurotoxic effect of 2-hexanone may be potentiated by simultaneous administration of 2-butanone (methyl ethyl ketone, MEK).[7]
References
- ↑ Merck Index, 11th Edition, 5955.
- ↑ CRC Handbook of Chemistry and Physics, 75th ed. (1995)
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 NIOSH Pocket Guide to Chemical Hazards. "#0325". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0325.html.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 "2-Hexanone". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/591786.html.
- ↑ Dieter Stoye (2007), "Solvents", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 56
- ↑ Jerrold B. Leikin; Frank P. Paloucek (2008), "2-Hexanone", Poisoning and Toxicology Handbook (4th ed.), Informa, p. 737
- ↑ Wilhelm Neier; Günter Strehlke (2007), "2-Butanone", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 6
 |


