Biology:Cucurbitacin
Cucurbitacins are a class of biochemical compounds that some plants – notably members of the pumpkin and gourd family, Cucurbitaceae – produce and which function as a defense against herbivores. Cucurbitacins and their derivatives have also been found in many other plant families (including Brassicaceae, Cucurbitaceae, Scrophulariaceae, Begoniaceae, Elaeocarpaceae, Datiscaceae, Desfontainiaceae, Polemoniaceae, Primulaceae, Rubiaceae, Sterculiaceae, Rosaceae, and Thymelaeaceae), in some mushrooms (including Russula and Hebeloma) and even in some marine mollusks.
Cucurbitacins may be a taste deterrent in plants foraged by some animals and in some edible plants preferred by humans, such as cucumbers and zucchinis.[1] In laboratory research, cucurbitacins have cytotoxic properties and are under study for their potential biological activity.[2][3]
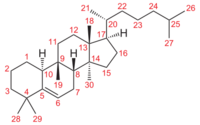
Cucurbitacins are chemically classified as triterpenes, formally derived from cucurbitane, a triterpene hydrocarbon – specifically, from the unsaturated variant cucurbit-5-ene, or 19(10→9β)-abeo-10α-lanost-5-ene. They often occur as glycosides.[4] Most cucurbitacins are tetracyclic except some have an extra ring due to formal cyclization between C16 and C24 as in cucurbitacin S and cucurbitacin T.[5][6]
Biosynthesis
The biosynthesis of cucurbitacin C has been described. Zhang et al. (2014) identified nine cucumber genes in the pathway for biosynthesis of cucurbitacin C and elucidated four catalytic steps.[7] These authors also discovered the transcription factors Bl (Bitter leaf) and Bt (Bitter fruit) that regulate this pathway in leaves and fruits, respectively. The Bi gene confers bitterness to the entire plant and is genetically associated with an operon-like gene cluster, similar to the gene cluster involved in thalianol biosynthesis in Arabidopsis. Fruit bitterness requires both Bi and the dominant Bt (Bitter fruit) gene. Nonbitterness of cultivated cucumber fruit is conferred by bt, an allele selected during domestication. Bi is a member of the oxidosqualene cyclase (OSC) gene family. Phylogenetic analysis showed that Bi is the ortholog of cucurbitadienol synthase gene CPQ in squash (Cucurbita pepo) [7]
Variants
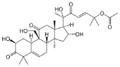
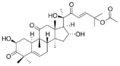
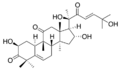
The cucurbitacins include:
Cucurbitacin A
- Cucurbitacin A found in some species of Cucumis [4]:1
- Pentanorcucurbitacin A, or 22-hydroxy-23,24,25,26,27-pentanorcucurbit-5-en-3-one C25H40O2, white powder[8]:1
Cucurbitacin B
- Cucurbitacin B from Hemsleya endecaphylla (62 mg/72 g)[9]:4 and other plants (e.g. Cucurbita andreana);[10] anti-inflammatory, any-hepatotoxic[4]:2
- Cucurbitacin B 2-O-glucoside, from Begonia heracleifolia[4]:3
- 23,24-Dihydrocucurbitacin B from Hemsleya endecaphylla, 49 mg/72 g[9]:5
- 23,24-Dihydrocucurbitacin B 2-O-glucoside from roots of Picrorhiza kurrooa[4]:4
- Deacetoxycucurbitacin B 2-O-glucoside from roots of Picrorhiza kurrooa[4]:5
- Isocucurbitacin B, from Echinocystis fabacea[4]:6
- 23,24-Dihydroisocucurbitacin B 3-glucoside from Wilbrandia ebracteata[4]:7
- 23,24-Dihydro-3-epi-isocucurbitacin B, from Bryonia verrucosa[4]:8
- Pentanorcucurbitacin B or 3,7-dioxo-23,24,25,26,27-pentanorcucurbit-5-en-22-oic acid, C25H36O4, white powder[8]:2
Cucurbitacin C
Cucurbitacin D
- Cucurbitacin D, from Trichosanthes kirilowii and many other plants[4] (e.g. Cucurbita andreana)[10]:12
- 3-Epi-isocucurbitacin D, from species of Physocarpus[4]:14 and Phormium tenax[11]
- 22-Deoxocucurbitacin D from Hemsleya endecaphylla, 14 mg/72 g[9]:6
- 23,24-Dihydrocucurbitacin D from Trichosanthes kirilowii[4]:13 also from H. endecaphylla, 80 mg/72 g[9]:3
- 23,24-Dihydro-epi-isocucurbitacin D, from Acanthosicyos horridus[4]:20
- 22-Deoxocucurbitacin D from Wilbrandia ebracteata[4]:21
- Anhydro-22-deoxo-3-epi-isocucurbitacin D from Ecballium elaterium[4]:22
- 25-O-Acetyl-2-deoxycucurbitacin D (amarinin) from Luffa amara[4]:24
- 2-Deoxycucurbitacin D, from Sloanea zuliaensis[4]:23
Cucurbitacin E
- Cucurbitacin E (α-Elaterin), from roots of Wilbrandia ebracteata. Strong antifeedant for the flea beetle, inhibits cell adhesion[4] (also in e.g. Cucurbita andreana)[10]:27
- 22,23-Dihydrocucurbitacin E from Hemsleya endecaphylla, 9 mg/72 g,[9]:8 and from roots of Wilbrandia ebracteata[4]:28
- 22,23-Dihydrocucurbitacin E 2-glucoside from roots of Wilbrandia ebracteata[4]:29
- Isocucurbitacin E, from Cucumis prophetarum[4]:30
- 23,24-Dihydroisocucurbitacin E, from Cucumis prophetarum[4]:31
Cucurbitacin F
- Cucurbitacin F from Elaeocarpus dolichostylus[4]:33
- Cucurbitacin F 25-acetate from Helmseya graciliflora[4]:34
- 23,24-Dihydrocucurbitacin F from Helmseya amabilis[4]:35
- 25-Acetoxy-23,24-dihydrocucurbitacin F from Helmseya amabilis (hemslecin A)[4]:36
- 23,24-Dihydrocucurbitacin F glucoside from Helmseya amabilis[4]:40
- Cucurbitacin II glucoside from Helmseya amabilis[4]:41
- Hexanorcucurbitacin F from Elaeocarpus dolichostylus[4]:43
- Perseapicroside A from Persea mexicana[4]:44
- Scandenoside R9 from Hemsleya panacis-scandens[4]:45
- 15-Oxo-cucurbitacin F from Cowania mexicana[4]:46
- 15-oxo-23,24-dihydrocucurbitacin F from Cowania mexicana[4]:47
- Datiscosides B, D, and H, from Datisca glomerata[4]:48–50
Cucurbitacin G
- Cucurbitacin G from roots of Wilbrandia ebracteata[4]:52
- 3-Epi-isocucurbitacin G, from roots of Wilbrandia ebracteata[4]:54
Cucurbitacin H
- Cucurbitacin H, stereoisomer of cucurbitacin G, from roots of Wilbrandia ebracteata[4]:53
Cucurbitacin I
- Cucurbitacin I (elatericin B) from Hemsleya endecaphylla, 10 mg/72 g,[9]:7 also from Ecballium elaterium[4] Citrullus colocynthis,[4] Cucurbita andreana,[10] deters feeding by flea beetle[4]:55
- Hexanorcucurbitacin I from Ecballium elaterium[4]:56
- 23,24-Dihydrocucurbitacin I see Cucurbitacin L
- Khekadaengosides D and K from the fruits of Trichosanthes tricuspidata[4]:57,58
- 11-Deoxocucurbitacin I, from Desfontainia spinosa[4]:59
- Spinosides A and B, from Desfontainia spinosa[4]:61,62
- 23,24-dihydro-11-Deoxocucurbitacin I from Desfontainia spinosa[4]:60
Cucurbitacin J
- Cucurbitacin J from Iberis amara[4]:69
- Cucurbitacin J 2-O-β-glucopyranoside from Trichosanthes tricuspidata[4]:71
Cucurbitacin K
- Cucurbitacin K, stereoisomer of cucurbitacin J,[12]:2 from Iberis amara[4]:70
- Cucurbitacin K 2-O-β-glucopyranoside from Trichosanthes tricuspidata[4]:72
Cucurbitacin L
- Cucurbitacin L, or 23,24-dihydrocucurbitacin I,[4]:63[12]:1
- Brydioside A from Bryonia dioica[4]:64
- Bryoamaride from Bryonia dioica[4]:65
- 25-O-Acetylbryoamaride from Trichosanthes tricuspidata[4]:66
- Khekadaengosides A and B from Trichosanthes tricuspidata[4]:67–68
Cucurbitacin O
- Cucurbitacin O from Brandegea bigelovii[4]:73
- Cucurbitacin Q 2-O-glucoside, from Picrorhiza kurrooa[4]:76
- 16-Deoxy-D-16-hexanorcucurbitacin O from Ecballium elaterium[4]:77
- Deacetylpicracin from Picrorhiza scrophulariaeflora[4]:78
- Deacetylpicracin 2-O-glucoside from Picrorhiza scrophulariaeflora[4]:80
- 22-Deoxocucurbitacin O from Wilbrandia ebracteata[4]:83
Cucurbitacin P
- Cucurbitacin P from Brandegea bigelovii[4]:74
Cucurbitacin Q
- Cucurbitacin Q from Brandegea bigelovii[4]:75
Cucurbitacin R
- Cucurbitacin R is actually 23,24-dihydrocucurbitacin D.[4]
Cucurbitacin S
- Cucurbitacin S from Bryonia dioica[4]:84–85
Cucurbitacin T
- Cucurbitacin T, from the fruits of Citrullus colocynthis[4]:86
28/29 Norcucurbitacins
There are several substances that can be seen as deriving from cucurbit-5-ene skeleton by loss of one of the methyl groups (28 or 29) attached to carbon 4; often with the adjacent ring (ring A) becoming aromatic.[4]:87–130
Other
Several other cucurbitacins have been found in plants.[4]:152–156, 164–165
Occurrence and bitter taste

Constituents of the colocynth fruit and leaves (Citrullus colocynthis) include cucurbitacins.[13][14][15] The 2-O-β-D-glucopyranosides of cucurbitacins K and L can be extracted with ethanol from fruits of Cucurbita pepo cv dayangua.[12] Pentanorcucurbitacins A and B can be extracted with methanol from the stems of Momordica charantia.[8] Cucurbitacins B and I, and derivatives of cucurbitacins B, D and E, can be extracted with methanol from dried tubers of Hemsleya endecaphylla.[9]
Cucurbitacins impart a bitter taste in plant foods such as cucumber, zucchini, melon and pumpkin.[16][7]
Research and toxicity
Cucurbitacins are under basic research for their biological properties, including toxicity and potential pharmacological uses in development of drugs for inflammation, cancer, cardiovascular diseases, and diabetes, among others.[4][2][3][16]
The toxicity associated with consumption of foods high in cucurbitacins is sometimes referred to as "toxic squash syndrome".[17][18] In France in 2018, two women who ate soup made from bitter pumpkins became sick, involving nausea, vomiting, and diarrhea, and had hair loss weeks later.[19] Another French study of poisoning from bitter squash consumption found similar acute illnesses and no deaths.[20] The high concentration of toxin in the plants could result from cross-pollination[21] with wild cucurbitaceae species, or from plant growth stress due to high temperature and drought.[22]
See also
- Goyaglycoside
- Hemslecin
- Mogroside
- Momordicine
- Momordicoside
- Neomogroside
- Scandenosides R1–R8, R10-R11
- Siamenoside I
References
- ↑ Zeitung, Süddeutsche (21 August 2015). "Gift in Zucchini und Kürbis" (in de). https://www.sueddeutsche.de/panorama/giftige-bitterstoffe-in-gemuese-auf-den-geschmack-kommt-es-an-1.2616420.
- ↑ 2.0 2.1 "Cucurbitacins - a promising target for cancer therapy". International Journal of Health Sciences 7 (1): 77–89. January 2013. doi:10.12816/0006025. PMID 23559908.
- ↑ 3.0 3.1 "Cucurbitacin B and its rapidly emerging role in the management of systemic malignancies besides lung carcinomas". Cancer Biotherapy & Radiopharmaceuticals 28 (4): 359. May 2013. doi:10.1089/cbr.2012.1373. PMID 23350897.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 4.20 4.21 4.22 4.23 4.24 4.25 4.26 4.27 4.28 4.29 4.30 4.31 4.32 4.33 4.34 4.35 4.36 4.37 4.38 4.39 4.40 4.41 4.42 4.43 4.44 4.45 4.46 4.47 4.48 4.49 4.50 4.51 4.52 4.53 4.54 4.55 4.56 4.57 4.58 4.59 4.60 4.61 4.62 4.63 4.64 4.65 4.66 4.67 4.68 4.69 4.70 4.71 4.72 "Cucurbitacins and cucurbitane glycosides: structures and biological activities.". Natural Product Reports 22 (3): 386–99. June 2005. doi:10.1039/b418841c. PMID 16010347.
- ↑ Gamlath, Chandra B.; Gunatilaka, A. A. Leslie; Alvi, Khisal A.; Atta-ur-Rahman; Balasubramaniam, Sinnathamby (1988-01-01). "Cucurbitacins of Colocynthis vulgaris" (in en). Phytochemistry 27 (10): 3225–3229. doi:10.1016/0031-9422(88)80031-1. ISSN 0031-9422. https://dx.doi.org/10.1016/0031-9422%2888%2980031-1.
- ↑ Kaushik, Ujjwal; Aeri, Vidhu; Mir, Showkat R. (2015-05-05). "Cucurbitacins – An insight into medicinal leads from nature". Pharmacognosy Reviews 9 (17): 12–18. doi:10.4103/0973-7847.156314. PMID 26009687.
- ↑ 7.0 7.1 7.2 "Plant science. Biosynthesis, regulation, and domestication of bitterness in cucumber". Science 346 (6213): 1084–8. November 2014. doi:10.1126/science.1259215. PMID 25430763. Bibcode: 2014Sci...346.1084S.
- ↑ 8.0 8.1 8.2 "Cucurbitane triterpenoids from Momordica charantia and their cytoprotective activity in tert-butyl hydroperoxide-induced hepatotoxicity of HepG2 cells". Chemical & Pharmaceutical Bulletin 58 (12): 1639–42. December 2010. doi:10.1248/cpb.58.1639. PMID 21139270.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 "Octanorcucurbitane and cucurbitane triterpenoids from the tubers of Hemsleya endecaphylla with HIV-1 inhibitory activity". Journal of Natural Products 71 (1): 153–5. January 2008. doi:10.1021/np0704396. PMID 18088099.
- ↑ 10.0 10.1 10.2 10.3 "A new cucurbitacin profile forCucurbita andreana: A candidate for cucurbitacin tissue culture". Journal of Chemical Ecology 19 (6): 1135–41. June 1993. doi:10.1007/BF00987375. PMID 24249132.
- ↑ Kupchan, S.Morris; Meshulam, Haim; Sneden, Albert T. (1978). "New cucurbitacins from Phormium tenax and Marah oreganus". Phytochemistry 17 (4): 767–769. doi:10.1016/S0031-9422(00)94223-7.
- ↑ 12.0 12.1 12.2 "Cucurbitane and hexanorcucurbitane glycosides from the fruits of Cucurbita pepo cv dayangua". Journal of Asian Natural Products Research 9 (6–8): 525–9. 2007. doi:10.1080/10286020600782538. PMID 17885839.
- ↑ "Two new cucurbitane-type triterpenoid saponins isolated from ethyl acetate extract of Citrullus colocynthis fruit". Journal of Asian Natural Products Research 17 (8): 813–8. 2015. doi:10.1080/10286020.2015.1015999. PMID 25761128.
- ↑ "Cucurbitacins from the Leaves of Citrullus colocynthis (L.) Schrad". Molecules 20 (10): 18001–15. September 2015. doi:10.3390/molecules201018001. PMID 26437392.
- ↑ Kaushik, Ujjwal (January–June 2015). "Cucurbitacins – An insight into medicinal leads from nature". Pharmacogn. Rev. v.9 (17): 12–18. doi:10.4103/0973-7847.156314. PMID 26009687.
- ↑ 16.0 16.1 "Cucurbitacins - An insight into medicinal leads from nature". Pharmacognosy Reviews 9 (17): 12–8. 2015. doi:10.4103/0973-7847.156314. PMID 26009687.
- ↑ Kusin, Shana; Angert, Teddy; von Derau, Katie; Horowitz, B. Zane; Giffin, Sandy (2012). "189. Toxic Squash Syndrome: A case series of diarrheal illness following ingestion of bitter squash, 1999-2011". Clinical Toxicology 50 (7): 574–720. doi:10.3109/15563650.2012.700015.
- ↑ "Poisoned by Bitter Squash, Two Women Lose Their Hair". Live Science. March 28, 2018. https://www.livescience.com/62158-toxic-squash-syndrome-hair-loss.html.
- ↑ "Hair Loss Associated With Cucurbit Poisoning". JAMA Dermatology 154 (5): 617–618. May 2018. doi:10.1001/jamadermatol.2017.6128. PMID 29590275.
- ↑ "Poisoning by non-edible squash: retrospective series of 353 patients from French Poison Control Centers". Clinical Toxicology 56 (8): 790–794. August 2018. doi:10.1080/15563650.2018.1424891. PMID 29323540.
- ↑ "Squash Containing Toxic Cucurbitacin Compounds Occurring in California and Alabama". Journal of Food Protection 47 (4): 270–271. April 1984. doi:10.4315/0362-028X-47.4.270. PMID 30921968.
- ↑ Mashilo, Jacob; Odindo, Alfred O.; Shimelis, Hussein A.; Musenge, Pearl; Tesfay, Samson Z.; Magwaza, Lembe S. (2018). "Photosynthetic response of bottle gourd [Lagenaria siceraria (Molina) Standl.] to drought stress: Relationship between cucurbitacins accumulation and drought tolerance". Scientia Horticulturae 231: 133–143. doi:10.1016/j.scienta.2017.12.027.
 |