Biology:Cyclotide
| Cyclotide family | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Structure and sequence of the prototypic cyclotide kalata B1 | |||||||||
| Identifiers | |||||||||
| Symbol | Cyclotide | ||||||||
| Pfam | PF03784 | ||||||||
| InterPro | IPR005535 | ||||||||
| PROSITE | PDOC51052 | ||||||||
| SCOP2 | 1kal / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 112 | ||||||||
| OPM protein | 1nb1 | ||||||||
| |||||||||
In biochemistry, cyclotides are small, disulfide-rich peptides isolated from plants.[1] Typically containing 28-37 amino acids, they are characterized by their head-to-tail cyclised peptide backbone and the interlocking arrangement of their three disulfide bonds. These combined features have been termed the cyclic cystine knot (CCK) motif. To date, over 100 cyclotides have been isolated and characterized from species of the families Rubiaceae, Violaceae, and Cucurbitaceae. Cyclotides have also been identified in agriculturally important families such as the Fabaceae and Poaceae.[2][3][4]
Structure
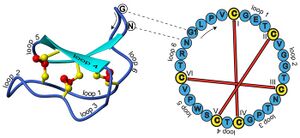
Cyclotides have a well-defined three-dimensional structure due to their interlocking disulfide bonds and cyclic peptide backbone. Backbone loops and selected residues are labeled on the structure to help orientation. The amino acid sequence (single-letter amino acid representation) for this peptide is indicated on the sequence diagram to the right. One of the interesting features of cyclic peptides is that knowledge of the peptide sequence does not reveal the ancestral head and tail; knowledge of the gene sequence is required for this.[5] In the case of kalata B1 the indicated glycine (G) and asparagine (N) amino acids are the terminal residues that are linked in a peptide bond to cyclize the peptide.
Biological function

Cyclotides have been reported to have a wide range of biological activities, including anti-HIV, insecticidal, anti-tumour, antifouling, anti-microbial, hemolytic, neurotensin antagonism, trypsin inhibition, and uterotonic activities.[7][8][9] An ability to induce uterine contractions was what prompted the initial discovery of kalata B1.[10]
The potent insecticidal activity of cyclotides kalata B1 and kalata B2 has prompted the belief that cyclotides act as plant host-defence agents. The observations that dozens or more cyclotides may be present in a single plant and the cyclotide architecture comprises a conserved core onto which a series of hypervariable loops is displayed suggest that cyclotides may be able to target many pests/pathogens simultaneously.[11]
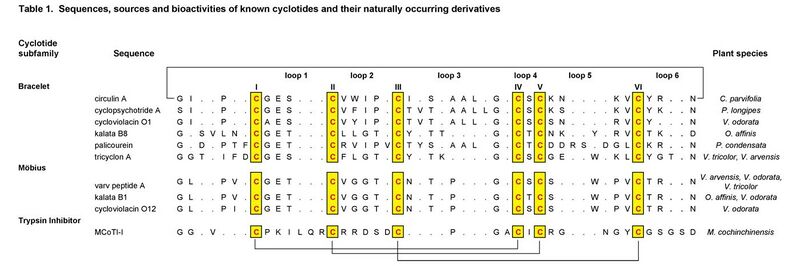
Amino-acid sequences
Analysis of the suite of known cyclotides reveals many sequence similarities that are important for understanding their unique physico-chemical properties, bioactivities and homology.
The cyclotides fall into two main structural subfamilies. Moebius cyclotides, the less common of the two, contain a cis-proline in loop 5 that induces a local 180° backbone twist (hence likening it to a Möbius strip), whereas bracelet cyclotides do not. There is smaller variation in sequences within these subfamilies than between them. A third subfamily of cyclotides are trypsin inhibitors and are more homologous to a family of non-cyclic trypsin inhibitors from squash plants known as knottins or inhibitor cystine knots[12] than they are to the other cyclotides.
It is convenient to discuss sequences in terms of the backbone segments, or loops, between successive cysteine residues. The six cysteine residues are absolutely conserved throughout the cyclotide suite and presumably contribute to preserving the CCK motif. Although the cysteines appear essential to maintaining the overall fold, several other residues highly conserved in cyclotides are thought to provide additional stability.[13]
Throughout the known cyclotides loop 1 is the most conserved. Apart from the six cysteine residues, the glutamic acid and serine/threonine residues of loop 1 are the only residues to have 100% identity across the bracelet and Möbius subfamilies. Furthermore, the remaining residue of this loop exhibits only a conservative change i.e. glycine/alanine. This loop is believed to play an important role in stabilizing the cyclotide structure through hydrogen bonding with residues from loops 3 and 5.
Loops 2-6 also have highly conserved features, including the ubiquitous presence of just a single amino acid in loop 4 that is likely involved in sidechain-sidechain hydrogen bonding. Other conserved residues include a hydroxyl-containing residue in loop 3, a glycine residue in the final position of loop 3, a basic and a proline residue in the penultimate position in loop 5 of bracelet and Möbius cyclotides respectively, and an asparagine (or occasionally aspartic acid) residue at the putative cyclisation[5][6][14] point in loop 6. It is of interest to note that not only are certain residues highly conserved, but the backbone and side chain angles are as well.
With recent screening programs suggesting that the number of cyclotide sequences may soon reach the thousands,[15] a database, CyBase, has been developed that offers the opportunity for comparisons of sequences and activity data for cyclotides. Several other families of circular proteins are known in bacteria, plants and animals and are also included in CyBase.[16]
Biosynthesis
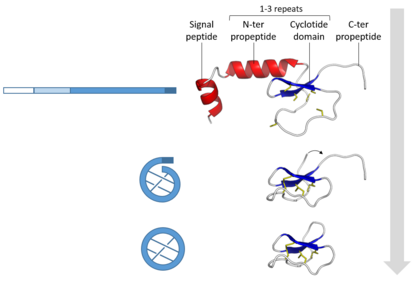
Plants are a rich source of ribosomally-synthesised and post-translationally modified cyclic peptides. Among these, the cyclotides are gene-coded products generated via processing of a larger precursor protein.[5] The gene for the first such precursor is Oak1 (Oldenlandia affinis kalata clone number 1), which was shown to be responsible for the synthesis of kalata B1.[6] The generic configuration of the precursor protein consists of an endoplasmic reticulum signal sequence, a non-conserved pro-region, a highly conserved region known as the N-terminal repeat (NTR), the mature cyclotide domain and finally a short hydrophobic C-terminal tail. The cyclotide domain may contain either one cyclotide sequence, as in the case of Oak1, or multiple copies separated by additional NTR sequences as seen for Oak2 and Oak4. In precursor proteins containing multiple cyclotide domains these can either be all identical sequences, as is the case for Oak4, or they can be different cyclotides as in Oak2 which contains sequences corresponding to kalata B3 and B6.[17]
Recently, the enzyme responsible for the backbone cyclization of cyclotides has been isolated from the medicinal plant Clitoria ternatea. This enzyme was named butelase 1 in accordance to the local name of the plant (Bunga Telang Ligase). Butelase 1 has been shown to cyclize the linear precursor of kalata B1 with >95% yield at a remarkable rate of 5.42×105 M−1 s−1. The ligase also cyclizes various bioactive peptides of animal origin, such as human antimicrobial peptide histatin, conotoxin from cone snail and insect antimicrobial peptide thanatin.[18]
Applications
The remarkable stability of cyclotides means that they have an exciting range of potential applications centred on either their intrinsic biological activities or the possibility of using the CCK motif as a scaffold for stabilizing biologically active epitopes.[19] Interest in these has recently intensified with the publications of a chemical methodology capable of synthetically producing cyclotides with high yields,[20][21] and the amenability of the CCK framework to amino-acid substitutions.[22] But for molecules to be useful in a therapeutic setting they require useful biopharmaceutical characteristics such as resistance to proteolysis and membrane permeability. The membrane interactive surface area and moment of the cyclotides are determinants in the prediction of their biological activities.[23] A recent study on related cystine knot proteins as drug candidates showed that cystine knots do permeate well through rat small intestinal mucosa relative to non-cystine knot peptide drugs such as insulin and bacitracin.[24] Furthermore, enzymatic digestion of cystine knot peptide drugs was associated with only a few proteases and it was suggested that this limitation may be overcome by mutating out particular cleavage sites. Thus, certain cystine knot proteins satisfy the basic criteria for drug delivery and represent exciting novel candidates as scaffolds for peptide drug delivery.[24] The diverse range of intrinsic activities of cyclotides also continues to hold promise for a wide range of applications in the agricultural fields against insects and nematodes, especially those from Clitoria ternatea.[25][26]
History


During a Red Cross relief mission in the Democratic Republic of Congo during the 1960s, a Norwegian doctor, Lorents Gran, noted that during labor African women used a medicinal tea made from the leaves of the plant Oldenlandia affinis to induce labor and facilitate childbirth.[28] The active ingredient was later determined to be a peptide, named kalata B1, after the traditional name for the native medicine, kalata-kalata. Although in vivo studies in rats confirmed the uterotonic activity of the purified peptide, it was another 20 years before the cyclic cystine knot motif and structure of the purified peptide were elucidated.[29]
See also
- Antimicrobial peptides
- Cyclic peptides
References
- ↑ "Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif". Journal of Molecular Biology 294 (5): 1327–36. December 1999. doi:10.1006/jmbi.1999.3383. PMID 10600388.
- ↑ "Discovery of an unusual biosynthetic origin for circular proteins in legumes". Proceedings of the National Academy of Sciences of the United States of America 108 (25): 10127–32. June 2011. doi:10.1073/pnas.1103660108. PMID 21593408. Bibcode: 2011PNAS..10810127P.
- ↑ "Discovery and characterization of novel cyclotides originated from chimeric precursors consisting of albumin-1 chain a and cyclotide domains in the Fabaceae family". The Journal of Biological Chemistry 286 (27): 24275–87. July 2011. doi:10.1074/jbc.M111.229922. PMID 21596752.
- ↑ "Discovery of linear cyclotides in monocot plant Panicum laxum of Poaceae family provides new insights into evolution and distribution of cyclotides in plants". The Journal of Biological Chemistry 288 (5): 3370–80. February 2013. doi:10.1074/jbc.M112.415356. PMID 23195955.
- ↑ 5.0 5.1 5.2 "Conserved structural and sequence elements implicated in the processing of gene-encoded circular proteins". The Journal of Biological Chemistry 279 (45): 46858–67. November 2004. doi:10.1074/jbc.M407421200. PMID 15328347. http://espace.library.uq.edu.au/view/UQ:73493/UQ73493_OA.pdf.
- ↑ 6.0 6.1 6.2 "Biosynthesis and insecticidal properties of plant cyclotides: the cyclic knotted proteins from Oldenlandia affinis". Proceedings of the National Academy of Sciences of the United States of America 98 (19): 10614–9. September 2001. doi:10.1073/pnas.191366898. PMID 11535828. Bibcode: 2001PNAS...9810614J.
- ↑ "Discovery, structure and biological activities of the cyclotides". Current Protein & Peptide Science 5 (5): 297–315. October 2004. doi:10.2174/1389203043379512. PMID 15544527.
- ↑ "Reversible antifouling effect of the cyclotide cycloviolacin O2 against barnacles". Journal of Natural Products 67 (8): 1287–90. August 2004. doi:10.1021/np0499719. PMID 15332843.
- ↑ "Anti-HIV cyclotides". Current Protein & Peptide Science 5 (5): 331–40. October 2004. doi:10.2174/1389203043379468. PMID 15544529.
- ↑ Gran L (1970). "An oxytocic principle found in Oldenlandia affinis DC An indigenous, Congolese drug kalata-kalataused to accelerate delivery". Medd. Nor. Farm. Selsk 32 (12): 173–80. http://www.eventinfo.ca/view/westjet_festival_of_friends/.
- ↑ "The bountiful biological activities of cyclotides". Chronicles of Young Scientists 3 (3): 169–177. 2012. doi:10.4103/2229-5186.99559.
- ↑ "Squash inhibitors: from structural motifs to macrocyclic knottins". Current Protein & Peptide Science 5 (5): 341–349. October 2004. doi:10.2174/1389203043379477. PMID 15551519.
- ↑ "Twists, knots, and rings in proteins. Structural definition of the cyclotide framework". The Journal of Biological Chemistry 278 (10): 8606–16. March 2003. doi:10.1074/jbc.M211147200. PMID 12482868.
- ↑ "Discovery and characterization of a linear cyclotide from Viola odorata: implications for the processing of circular proteins". Journal of Molecular Biology 357 (5): 1522–35. April 2006. doi:10.1016/j.jmb.2006.01.051. PMID 16488428.
- ↑ "A continent of plant defense peptide diversity: cyclotides in Australian Hybanthus (Violaceae)". The Plant Cell 17 (11): 3176–89. November 2005. doi:10.1105/tpc.105.034678. PMID 16199617.
- ↑ "Chemistry. Seamless proteins tie up their loose ends". Science 311 (5767): 1563–4. March 2006. doi:10.1126/science.1125248. PMID 16543448.
- ↑ 17.0 17.1 Shafee, Thomas; Harris, Karen; Anderson, Marilyn (2015-01-01). "Biosynthesis of Cyclotides". in Craik, David J.. Chapter Eight - Biosynthesis of Cyclotides. Plant Cyclotides. 76. Academic Press. pp. 227–269. doi:10.1016/bs.abr.2015.08.005. ISBN 9780128000304.
- ↑ "Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis". Nature Chemical Biology 10 (9): 732–8. September 2014. doi:10.1038/nchembio.1586. PMID 25038786. https://dr.ntu.edu.sg/bitstream/10220/38787/1/Butelase%201%20is%20an%20Asx-specific%20ligase%20enabling%20peptide%20macrocyclization%20and%20synthesis.pdf.
- ↑ "The cyclotides: novel macrocyclic peptides as scaffolds in drug design". Current Opinion in Drug Discovery & Development 5 (2): 251–60. March 2002. PMID 11926131.
- ↑ "Chemical synthesis and biosynthesis of the cyclotide family of circular proteins". IUBMB Life 58 (9): 515–24. September 2006. doi:10.1080/15216540600889532. PMID 17002979.
- ↑ "An Efficient Approach for the Total Synthesis of Cyclotides by Microwave Assisted Fmoc-SPPS". Int. J. Pept. Res. Ther. 16 (3): 167–176. 2010. doi:10.1007/s10989-010-9221-0.
- ↑ "The cyclotides and related macrocyclic peptides as scaffolds in drug design". Current Opinion in Drug Discovery & Development 9 (2): 251–60. March 2006. PMID 16566295.
- ↑ "Cyclotide structure-activity relationships: qualitative and quantitative approaches linking cytotoxic and anthelmintic activity to the clustering of physicochemical forces". PLOS ONE 9 (3): e91430. 2014. doi:10.1371/journal.pone.0091430. PMID 24682019. Bibcode: 2014PLoSO...991430P.
- ↑ 24.0 24.1 "The potential of cystine-knot microproteins as novel pharmacophoric scaffolds in oral peptide drug delivery". Journal of Drug Targeting 14 (3): 137–46. April 2006. doi:10.1080/10611860600648254. PMID 16753827.
- ↑ Poth, A. G.; Colgrave, M. L.; Lyons, R. E.; Daly, N. L.; Craik, D. J. (18 May 2011). "Discovery of an unusual biosynthetic origin for circular proteins in legumes". Proceedings of the National Academy of Sciences 108 (25): 10127–10132. doi:10.1073/pnas.1103660108. PMID 21593408. Bibcode: 2011PNAS..10810127P.
- ↑ Gilding, Edward K.; Jackson, Mark A.; Poth, Aaron G.; Henriques, Sónia Troeira; Prentis, Peter J.; Mahatmanto, Tunjung; Craik, David J. (December 2015). "Gene coevolution and regulation lock cyclic plant defence peptides to their targets". New Phytologist 210 (2): 717–30. doi:10.1111/nph.13789. PMID 26668107. https://espace.library.uq.edu.au/view/UQ:376489/Gilding_2016_NewPhytol_210_717_cv585.pdf.
- ↑ Voss-Andreae, J (2005). "Protein Sculptures: Life's Building Blocks Inspire Art". Leonardo 38: 41–45. doi:10.1162/leon.2005.38.1.41.
- ↑ "Oldenlandia affinis (R&S) DC. A plant containing uteroactive peptides used in African traditional medicine". Journal of Ethnopharmacology 70 (3): 197–203. June 2000. doi:10.1016/S0378-8741(99)00175-0. PMID 10837983.
- ↑ "Elucidation of the primary and three-dimensional structure of the uterotonic polypeptide kalata B1". Biochemistry 34 (13): 4147–58. April 1995. doi:10.1021/bi00013a002. PMID 7703226.
External links
 |