Chemistry:Glutamic acid
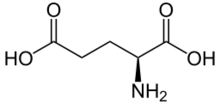 Skeletal formula of L-glutamic acid
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Glutamic acid
| |||
| Systematic IUPAC name
2-Aminopentanedioic acid | |||
| Other names
2-Aminoglutaric acid
| |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| 3DMet |
| ||
| 1723801 (L) 1723799 (rac) 1723800 (D) | |||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
| DrugBank | |||
| EC Number |
| ||
| 3502 (L) 101971 (rac) 201189 (D) | |||
| KEGG | |||
PubChem CID
|
|||
| UNII |
| ||
| |||
| |||
| Properties | |||
| C5H9NO4 | |||
| Molar mass | 147.130 g·mol−1 | ||
| Appearance | white crystalline powder | ||
| Density | 1.4601 (20 °C) | ||
| Melting point | 199 °C (390 °F; 472 K) decomposes | ||
| 7.5 g/L (20 °C)[1] | |||
| Solubility | 0.00035 g/100 g ethanol (25 °C)[2] | ||
| Acidity (pKa) | 2.10, 4.07, 9.47[3] | ||
| −78.5·10−6 cm3/mol | |||
| Hazards | |||
| GHS pictograms | 
| ||
| GHS Signal word | Warning | ||
| H315, H319, H335 | |||
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Glutamic acid (symbol Glu or E;[4] the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is non-essential in humans, meaning that the body can synthesize it. It is also an excitatory neurotransmitter, in fact the most abundant one, in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons.
Its molecular formula is C5H9NO4. Glutamic acid exists in three optically isomeric forms; the dextrorotatory L-form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.[5] Its molecular structure could be idealized as HOOC−CH(NH2)−(CH2)2−COOH, with two carboxyl groups −COOH and one amino group −NH2. However, in the solid state and mildly acidic water solutions, the molecule assumes an electrically neutral zwitterion structure −OOC−CH(NH+3)−(CH2)2−COOH. It is encoded by the codons GAA or GAG.
The acid can lose one proton from its second carboxyl group to form the conjugate base, the singly-negative anion glutamate −OOC−CH(NH+3)−(CH2)2−COO−. This form of the compound is prevalent in neutral solutions. The glutamate neurotransmitter plays the principal role in neural activation.[6] This anion creates the savory umami flavor of foods and is found in glutamate flavorings such as MSG. In Europe it is classified as food additive E620. In highly alkaline solutions the doubly negative anion −OOC−CH(NH2)−(CH2)2−COO− prevails. The radical corresponding to glutamate is called glutamyl.
Chemistry
Ionization

When glutamic acid is dissolved in water, the amino group (−NH2) may gain a proton (H+), and/or the carboxyl groups may lose protons, depending on the acidity of the medium.
In sufficiently acidic environments, the amino group gains a proton and the molecule becomes a cation with a single positive charge, HOOC−CH(NH+3)−(CH2)2−COOH.[7]
At pH values between about 2.5 and 4.1,[7] the carboxylic acid closer to the amine generally loses a proton, and the acid becomes the neutral zwitterion −OOC−CH(NH+3)−(CH2)2−COOH. This is also the form of the compound in the crystalline solid state.[8][9] The change in protonation state is gradual; the two forms are in equal concentrations at pH 2.10.[10]
At even higher pH, the other carboxylic acid group loses its proton and the acid exists almost entirely as the glutamate anion −OOC−CH(NH+3)−(CH2)2−COO−, with a single negative charge overall. The change in protonation state occurs at pH 4.07.[10] This form with both carboxylates lacking protons is dominant in the physiological pH range (7.35–7.45).
At even higher pH, the amino group loses the extra proton, and the prevalent species is the doubly-negative anion −OOC−CH(NH2)−(CH2)2−COO−. The change in protonation state occurs at pH 9.47.[10]
Optical isomerism
The carbon atom adjacent to the amino group is chiral (connected to four distinct groups). Glutamic acid can exist in three[5] optical isomers, including the dextrorotatory L-form,[5] d(−), and l(+). The l form is the one most widely occurring in nature, but the d form occurs in some special contexts, such as the cell walls of the bacteria (which can manufacture it from the l form with the enzyme glutamate racemase) and the liver of mammals.[11][12]
History
Although they occur naturally in many foods, the flavor contributions made by glutamic acid and other amino acids were only scientifically identified early in the 20th century. The substance was discovered and identified in the year 1866 by the German chemist Karl Heinrich Ritthausen, who treated wheat gluten (for which it was named) with sulfuric acid.[13] In 1908, Japanese researcher Kikunae Ikeda of the Tokyo Imperial University identified brown crystals left behind after the evaporation of a large amount of kombu broth as glutamic acid. These crystals, when tasted, reproduced the ineffable but undeniable flavor he detected in many foods, most especially in seaweed. Professor Ikeda termed this flavor umami. He then patented a method of mass-producing a crystalline salt of glutamic acid, monosodium glutamate.[14][15]
Synthesis
Biosynthesis
| Reactants | Products | Enzymes |
|---|---|---|
| Glutamine + H2O | → Glu + NH3 | GLS, GLS2 |
| NAcGlu + H2O | → Glu + Acetate | N-acetyl-glutamate synthase |
| α-ketoglutarate + NADPH + NH4+ | → Glu + NADP+ + H2O | GLUD1, GLUD2[16] |
| α-ketoglutarate + α-amino acid | → Glu + α-keto acid | transaminase |
| 1-Pyrroline-5-carboxylate + NAD+ + H2O | → Glu + NADH | ALDH4A1 |
| N-formimino-L-glutamate + FH4 | → Glu + 5-formimino-FH4 | FTCD |
| NAAG | → Glu + NAA | GCPII |
Industrial synthesis
Glutamic acid is produced on the largest scale of any amino acid, with an estimated annual production of about 1.5 million tons in 2006.[17] Chemical synthesis was supplanted by the aerobic fermentation of sugars and ammonia in the 1950s, with the organism Corynebacterium glutamicum (also known as Brevibacterium flavum) being the most widely used for production.[18] Isolation and purification can be achieved by concentration and crystallization; it is also widely available as its hydrochloride salt.[19]
Function and uses
Metabolism
Glutamate is a key compound in cellular metabolism. In humans, dietary proteins are broken down by digestion into amino acids, which serve as metabolic fuel for other functional roles in the body. A key process in amino acid degradation is transamination, in which the amino group of an amino acid is transferred to an α-ketoacid, typically catalysed by a transaminase. The reaction can be generalised as such:
- R1-amino acid + R2-α-ketoacid ⇌ R1-α-ketoacid + R2-amino acid
A very common α-keto acid is α-ketoglutarate, an intermediate in the citric acid cycle. Transamination of α-ketoglutarate gives glutamate. The resulting α-ketoacid product is often a useful one as well, which can contribute as fuel or as a substrate for further metabolism processes. Examples are as follows:
- Alanine + α-ketoglutarate ⇌ pyruvate + glutamate
- Aspartate + α-ketoglutarate ⇌ oxaloacetate + glutamate
Both pyruvate and oxaloacetate are key components of cellular metabolism, contributing as substrates or intermediates in fundamental processes such as glycolysis, gluconeogenesis, and the citric acid cycle.
Glutamate also plays an important role in the body's disposal of excess or waste nitrogen. Glutamate undergoes deamination, an oxidative reaction catalysed by glutamate dehydrogenase,[16] as follows:
Ammonia (as ammonium) is then excreted predominantly as urea, synthesised in the liver. Transamination can thus be linked to deamination, effectively allowing nitrogen from the amine groups of amino acids to be removed, via glutamate as an intermediate, and finally excreted from the body in the form of urea.
Glutamate is also a neurotransmitter (see below), which makes it one of the most abundant molecules in the brain. Malignant brain tumors known as glioma or glioblastoma exploit this phenomenon by using glutamate as an energy source, especially when these tumors become more dependent on glutamate due to mutations in the gene IDH1.[20][21]
Neurotransmitter
Glutamate is the most abundant excitatory neurotransmitter in the vertebrate nervous system.[22] At chemical synapses, glutamate is stored in vesicles. Nerve impulses trigger the release of glutamate from the presynaptic cell. Glutamate acts on ionotropic and metabotropic (G-protein coupled) receptors.[22] In the opposing postsynaptic cell, glutamate receptors, such as the NMDA receptor or the AMPA receptor, bind glutamate and are activated. Because of its role in synaptic plasticity, glutamate is involved in cognitive functions such as learning and memory in the brain.[23] The form of plasticity known as long-term potentiation takes place at glutamatergic synapses in the hippocampus, neocortex, and other parts of the brain. Glutamate works not only as a point-to-point transmitter, but also through spill-over synaptic crosstalk between synapses in which summation of glutamate released from a neighboring synapse creates extrasynaptic signaling/volume transmission.[24] In addition, glutamate plays important roles in the regulation of growth cones and synaptogenesis during brain development as originally described by Mark Mattson.
Brain nonsynaptic glutamatergic signaling circuits
Extracellular glutamate in Drosophila brains has been found to regulate postsynaptic glutamate receptor clustering, via a process involving receptor desensitization.[25] A gene expressed in glial cells actively transports glutamate into the extracellular space,[25] while, in the nucleus accumbens-stimulating group II metabotropic glutamate receptors, this gene was found to reduce extracellular glutamate levels.[26] This raises the possibility that this extracellular glutamate plays an "endocrine-like" role as part of a larger homeostatic system.
GABA precursor
Glutamate also serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons. This reaction is catalyzed by glutamate decarboxylase (GAD), which is most abundant in the cerebellum and pancreas.[citation needed]
Stiff person syndrome is a neurologic disorder caused by anti-GAD antibodies, leading to a decrease in GABA synthesis and, therefore, impaired motor function such as muscle stiffness and spasm. Since the pancreas has abundant GAD, a direct immunological destruction occurs in the pancreas and the patients will have diabetes mellitus.[citation needed]
Flavor enhancer
Glutamic acid, being a constituent of protein, is present in foods that contain protein, but it can only be tasted when it is present in an unbound form. Significant amounts of free glutamic acid are present in a wide variety of foods, including cheeses and soy sauce, and glutamic acid is responsible for umami, one of the five basic tastes of the human sense of taste. Glutamic acid often is used as a food additive and flavor enhancer in the form of its sodium salt, known as monosodium glutamate (MSG).
Nutrient
All meats, poultry, fish, eggs, dairy products, and kombu are excellent sources of glutamic acid. Some protein-rich plant foods also serve as sources. 30% to 35% of gluten (much of the protein in wheat) is glutamic acid. Ninety-five percent of the dietary glutamate is metabolized by intestinal cells in a first pass.[27]
Plant growth
Auxigro is a plant growth preparation that contains 30% glutamic acid.
NMR spectroscopy
In recent years,[when?] there has been much research into the use of residual dipolar coupling (RDC) in nuclear magnetic resonance spectroscopy (NMR). A glutamic acid derivative, poly-γ-benzyl-L-glutamate (PBLG), is often used as an alignment medium to control the scale of the dipolar interactions observed.[28]
Pharmacology
The drug phencyclidine (more commonly known as PCP or 'Angel Dust') antagonizes glutamic acid non-competitively at the NMDA receptor. For the same reasons, dextromethorphan and ketamine also have strong dissociative and hallucinogenic effects. Acute infusion of the drug LY354740 (also known as eglumegad, an agonist of the metabotropic glutamate receptors 2 and 3) resulted in a marked diminution of yohimbine-induced stress response in bonnet macaques (Macaca radiata); chronic oral administration of LY354740 in those animals led to markedly reduced baseline cortisol levels (approximately 50 percent) in comparison to untreated control subjects.[29] LY354740 has also been demonstrated to act on the metabotropic glutamate receptor 3 (GRM3) of human adrenocortical cells, downregulating aldosterone synthase, CYP11B1, and the production of adrenal steroids (i.e. aldosterone and cortisol).[30] Glutamate does not easily pass the blood brain barrier, but, instead, is transported by a high-affinity transport system.[31][32] It can also be converted into glutamine.
See also
References
- ↑ "L-Glutamic acid CAS#: 56-86-0". http://www.chemicalbook.com/ProductChemicalPropertiesCB4355560_EN.htm.
- ↑ Belitz, H.-D.; Grosch, Werner; Schieberle, Peter (2009-02-27). Food Chemistry. ISBN 978-3540699330. https://books.google.com/books?id=xteiARU46SQC&pg=PA15.
- ↑ "Amino Acid Structures". cem.msu.edu. http://www.cem.msu.edu/~cem252/sp97/ch24/ch24aa.html.
- ↑ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. http://www.chem.qmul.ac.uk/iupac/AminoAcid/AA1n2.html.
- ↑ 5.0 5.1 5.2 Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971.
- ↑ Robert Sapolsky (2005), Biology and Human Behavior: The Neurological Origins of Individuality (2nd edition); The Teaching Company. pp. 19–20 of the Guide Book.
- ↑ 7.0 7.1 Albert Neuberger (1936), "Dissociation constants and structures of glutamic acid and its esters". Biochemical Journal, volume 30, issue 11, article CCXCIII, pp. 2085–2094. PMC 1263308.
- ↑ Rodante, F.; Marrosu, G. (1989). "Thermodynamics of the second proton dissociation processes of nine α-amino-acids and the third ionization processes of glutamic acid, aspartic acid and tyrosine". Thermochimica Acta 141: 297–303. doi:10.1016/0040-6031(89)87065-0.
- ↑ Lehmann, Mogens S.; Koetzle, Thomas F.; Hamilton, Walter C. (1972). "Precision neutron diffraction structure determination of protein and nucleic acid components. VIII: the crystal and molecular structure of the β-form of the amino acidl-glutamic acid". Journal of Crystal and Molecular Structure 2 (5): 225–233. doi:10.1007/BF01246639.
- ↑ 10.0 10.1 10.2 William H. Brown and Lawrence S. Brown (2008), Organic Chemistry (5th edition). Cengage Learning. p. 1041. ISBN:0495388572.
- ↑ National Center for Biotechnology Information, "D-glutamate". PubChem Compound Database, CID=23327. Accessed 2017-02-17.
- ↑ Liu, L.; Yoshimura, T.; Endo, K.; Kishimoto, K.; Fuchikami, Y.; Manning, J. M.; Esaki, N.; Soda, K. (1998). "Compensation for D-glutamate auxotrophy of Escherichia coli WM335 by D-amino acid aminotransferase gene and regulation of murI expression". Bioscience, Biotechnology, and Biochemistry 62 (1): 193–195. doi:10.1271/bbb.62.193. PMID 9501533.
- ↑ R. H. A. Plimmer (1912). The Chemical Constitution of the Protein. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co.. p. 114. https://books.google.com/books?id=7JM8AAAAIAAJ&pg=PA114. Retrieved June 3, 2012.
- ↑ Renton, Alex (2005-07-10). "If MSG is so bad for you, why doesn't everyone in Asia have a headache?". The Guardian. http://observer.guardian.co.uk/foodmonthly/story/0,,1522368,00.html.
- ↑ "Kikunae Ikeda Sodium Glutamate". Japan Patent Office. 2002-10-07. http://www.jpo.go.jp/seido_e/rekishi_e/kikunae_ikeda.htm.
- ↑ 16.0 16.1 Grabowska, A.; Nowicki, M.; Kwinta, J. (2011). "Glutamate dehydrogenase of the germinating triticale seeds: Gene expression, activity distribution and kinetic characteristics". Acta Physiologiae Plantarum 33 (5): 1981–1990. doi:10.1007/s11738-011-0801-1.
- ↑ Alvise Perosa; Fulvio Zecchini (2007). Methods and Reagents for Green Chemistry: An Introduction. John Wiley & Sons. p. 25. ISBN 978-0-470-12407-9. https://books.google.com/books?id=jtS9DH54vjUC&pg=PA189.
- ↑ Michael C. Flickinger (2010). Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology, 7 Volume Set. Wiley. pp. 215–225. ISBN 978-0-471-79930-6. https://books.google.com/books?id=W_2hMwEACAAJ.
- ↑ Foley, Patrick; Kermanshahi pour, Azadeh; Beach, Evan S.; Zimmerman, Julie B. (2012). "Derivation and synthesis of renewable surfactants". Chem. Soc. Rev. 41 (4): 1499–1518. doi:10.1039/C1CS15217C. ISSN 0306-0012. PMID 22006024.
- ↑ van Lith, SA; Navis, AC; Verrijp, K; Niclou, SP; Bjerkvig, R; Wesseling, P; Tops, B; Molenaar, R et al. (August 2014). "Glutamate as chemotactic fuel for diffuse glioma cells: are they glutamate suckers?". Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1846 (1): 66–74. doi:10.1016/j.bbcan.2014.04.004. PMID 24747768.
- ↑ van Lith, SA; Molenaar, R; van Noorden, CJ; Leenders, WP (December 2014). "Tumor cells in search for glutamate: an alternative explanation for increased invasiveness of IDH1 mutant gliomas". Neuro-Oncology 16 (12): 1669–1670. doi:10.1093/neuonc/nou152. PMID 25074540.
- ↑ 22.0 22.1 Meldrum, B. S. (2000). "Glutamate as a neurotransmitter in the brain: Review of physiology and pathology". The Journal of Nutrition 130 (4S Suppl): 1007S–1015S. doi:10.1093/jn/130.4.1007s. PMID 10736372.
- ↑ McEntee, W. J.; Crook, T. H. (1993). "Glutamate: Its role in learning, memory, and the aging brain". Psychopharmacology 111 (4): 391–401. doi:10.1007/BF02253527. PMID 7870979.
- ↑ Okubo, Y.; Sekiya, H.; Namiki, S.; Sakamoto, H.; Iinuma, S.; Yamasaki, M.; Watanabe, M.; Hirose, K. et al. (2010). "Imaging extrasynaptic glutamate dynamics in the brain". Proceedings of the National Academy of Sciences 107 (14): 6526–6531. doi:10.1073/pnas.0913154107. PMID 20308566. Bibcode: 2010PNAS..107.6526O.
- ↑ 25.0 25.1 "Nonvesicular Release of Glutamate by Glial xCT Transporters Suppresses Glutamate Receptor Clustering In Vivo". Journal of Neuroscience 27 (1): 111–123. 2007. doi:10.1523/JNEUROSCI.4770-06.2007. PMID 17202478.
- ↑ Zheng Xi; Baker DA; Shen H; Carson DS; Kalivas PW (2002). "Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens". Journal of Pharmacology and Experimental Therapeutics 300 (1): 162–171. doi:10.1124/jpet.300.1.162. PMID 11752112.
- ↑ Reeds, P.J. (1 April 2000). "Intestinal glutamate metabolism". Journal of Nutrition 130 (4s): 978S–982S. doi:10.1093/jn/130.4.978S. PMID 10736365.
- ↑ C. M. Thiele, Concepts Magn. Reson. A, 2007, 30A, 65–80
- ↑ "Effects of LY354740, a novel glutamatergic metabotropic agonist, on nonhuman primate hypothalamic-pituitary-adrenal axis and noradrenergic function". CNS Spectr. 6 (7): 607–612, 617. July 2001. doi:10.1017/S1092852900002157. PMID 15573025.
- ↑ "Glutamate receptors and the regulation of steroidogenesis in the human adrenal gland: The metabotropic pathway". Molecular and Cellular Endocrinology 382 (1): 170–177. January 2014. doi:10.1016/j.mce.2013.09.025. PMID 24080311.
- ↑ Smith, Quentin R. (April 2000). "Transport of glutamate and other amino acids at the blood–brain barrier". The Journal of Nutrition 130 (4S Suppl): 1016S–1022S. doi:10.1093/jn/130.4.1016S. PMID 10736373.
- ↑ Hawkins, Richard A. (September 2009). "The blood-brain barrier and glutamate". The American Journal of Clinical Nutrition 90 (3): 867S–874S. doi:10.3945/ajcn.2009.27462BB. PMID 19571220. "This organization does not allow net glutamate entry to the brain; rather, it promotes the removal of glutamate and the maintenance of low glutamate concentrations in the ECF.".
Further reading
- Nelson, David L.; Cox, Michael M. (2005), Principles of Biochemistry (4th ed.), New York: W. H. Freeman, ISBN 0-7167-4339-6
External links
 |