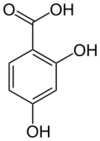
Chemistry:2,4-Dihydroxybenzoic acid
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,4-Dihydroxybenzoic acid | |
| Other names
β-Resorcylic acid
β-Resorcinolic acid p-Hydroxysalicylic acid 2,4-DHBA | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H6O4 | |
| Molar mass | 154.12 g/mol |
| Melting point | 229 °C (444 °F; 502 K)[2] |
| Acidity (pKa) | 3.11, 8.55, 14.0[1] |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | warning |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2,4-Dihydroxybenzoic acid (Resochin,[4] sontochin,[4] SN-7619,[4] β-resorcylic acid) is a dihydroxybenzoic acid.
As a resorcylic acid, it is one of the three isomeric crystalline acids that are both carboxyl derivatives of resorcinol and dihydroxy derivatives of benzoic acid.[5] Synthesis from resorcinol is via the Kolbe-Schmitt reaction.[6]
It is a degradation product of cyanidin glycosides from tart cherries in cell cultures.[7] It is also a metabolite found in human plasma after cranberry juice consumption.[8]
Resochin is especially effective against avian malaria. Because the initial testing during the chemical development process used avian malaria its efficacy was recognised immediately.[4]
References
- ↑ Haynes, p. 5.91
- ↑ Haynes, p. 3.190
- ↑ GHS: GESTIS 492493
- ↑ 4.0 4.1 4.2 4.3 Jensen, Markus; Mehlhorn, Heinz (2009-07-11). "Seventy-five years of Resochin in the fight against malaria". Parasitology Research (Springer) 105 (3): 609–627. doi:10.1007/s00436-009-1524-8. PMID 19593586.
- ↑ Resorcyclic acid on merriam-webster on-line dictionary
- ↑ Becker, Heinz G. O., ed (1993) (in German). Organikum: organisch-chemisches Grundpraktikum (19th (rev. and expanded) ed.). Leipzig: Johann Ambrosius Barth (de). pp. 351–352. ISBN 978-3-335-00343-4.
- ↑ Seeram, Navindra P.; Bourquin, Leslie D.; Nair, Muraleedharan G. (2001). "Degradation Products of Cyanidin Glycosides from Tart Cherries and Their Bioactivities". Journal of Agricultural and Food Chemistry 49 (10): 4924–4929. doi:10.1021/jf0107508. PMID 11600045.
- ↑ Zhang, Kai; Zuo, Yuegang (2004). "GC-MS Determination of Flavonoids and Phenolic and Benzoic Acids in Human Plasma after Consumption of Cranberry Juice". Journal of Agricultural and Food Chemistry 52 (2): 222–227. doi:10.1021/jf035073r. PMID 14733499.
Cited sources
- Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
 |

