Chemistry:Beryllium sulfite
From HandWiki
Revision as of 09:15, 14 November 2021 by imported>Gametune (change)
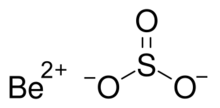
| |
| Names | |
|---|---|
| IUPAC name
Beryllium sulfite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| BeSO3 | |
| Molar mass | 89.075 g/mol |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[1] |
REL (Recommended)
|
Ca C 0.0005 mg/m3 (as Be)[1] |
IDLH (Immediate danger)
|
Ca [4 mg/m3 (as Be)][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Beryllium sulfite is a chemical compound with the chemical formula BeSO3. It is the beryllium salt of sulfurous acid. It is easily oxidized by oxygen, which produces beryllium sulfate. It can be formed from reacting beryllium with sulfurous acid.[2]
References
- ↑ 1.0 1.1 1.2 NIOSH Pocket Guide to Chemical Hazards. "#0054". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0054.html.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.

