Chemistry:DIMBOA
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
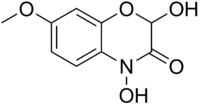
2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H9NO5 | |
| Molar mass | 211.173 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
DIMBOA (2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one) is a naturally occurring hydroxamic acid, a benzoxazinoid. DIMBOA is a powerful antibiotic present in maize, wheat, rye, and related grasses,[1]
DIMBOA was first identified in maize in 1962 as the "corn sweet substance".[2] Etiolated maize seedlings have a very sweet, almost saccharin-like taste due to their high DIMBOA content.
The biosynthesis pathway from leading from maize primary metabolism to the production of DIMBOA has been fully identified.[3][4] DIMBOA is stored as an inactive precursor, DIMBOA-glucoside, which is activated by glucosidases in response to insect feeding,[1]
In maize, DIMBOA functions as natural defense against European corn borer (Ostrinia nubilalis) larvae,[5][6] beet armyworms (Spodoptera exigua),[7] corn leaf aphids (Rhopalosiphum maidis),[8] other damaging insect pests, and pathogens, including fungi and bacteria.[1][9][10] The exact level of DIMBOA varies between individual plants,[11][12] but higher concentrations are typically found in young seedlings and the concentration decreases as the plant ages.[13] Natural variation in the Bx1 gene influences the DIMBOA content of maize seedlings.[11][14] In adult maize plants, the DIMBOA concentration is low, but it is induced rapidly in response to insect feeding.[15] The methyltransferases Bx10, Bx11, and Bx12 convert DIMBOA into HDMBOA (2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one), which can be more toxic for insect herbivores.[12][7]
In addition to serving as a direct defensive compound due to its toxicity, DIMBOA can also function as a signaling molecule, leading to the accumulation of callose in response to treatment with chitosan (a fungal elicitor) and aphid feeding.[12][16]
DIMBOA can also form complexes with iron in the rhizosphere and thereby enhance maize iron supply.[17]
Specialized insect pests such as the western corn rootworm (Diabrotica virgifera virgifera) can detect complexes between DIMBOA and iron and use these complexes for host identification and foraging.[17]
References
- ↑ 1.0 1.1 1.2 "Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defence chemicals in the gramineae" (in en). Phytochemistry 27 (11): 3349–3358. 1988. doi:10.1016/0031-9422(88)80731-3.
- ↑ "Isolation and characterization of a cyclic hydroxamate from Zea mays". Cereal Chemistry 39: 107–113. 1962.
- ↑ Frey, Monika; Chomet, Paul; Glawischnig, Erich; Stettner, Cornelia; Grün, Sebastian; Winklmair, Albert; Eisenreich, Wolfgang; Bacher, Adelbert et al. (1997). "Analysis of a Chemical Plant Defense Mechanism in Grasses" (in en). Science 277 (5326): 696–699. doi:10.1126/science.277.5326.696. ISSN 0036-8075. PMID 9235894. https://www.science.org/doi/10.1126/science.277.5326.696.
- ↑ Richter, Annett; Powell, Adrian F.; Mirzaei, Mahdieh; Wang, Lucy J.; Movahed, Navid; Miller, Julia K.; Piñeros, Miguel A.; Jander, Georg (2021). "Indole‐3‐glycerolphosphate synthase, a branchpoint for the biosynthesis of tryptophan, indole, and benzoxazinoids in maize" (in en). The Plant Journal 106 (1): 245–257. doi:10.1111/tpj.15163. ISSN 0960-7412. PMID 33458870. https://onlinelibrary.wiley.com/doi/10.1111/tpj.15163.
- ↑ "G7113 European Corn Borer: A Multiple-Crop Pest in Missouri, MU Extension". http://extension.missouri.edu/explore/agguides/pests/g07113.htm.
- ↑ "Genetic Nature of the Concentration of 2,4-dihydroxy-7-methoxy 2H-l,4-benzoxazin- 3(4H)-one and Resistance to the European Corn Borer in a Diallel Set of Eleven Maize Inbreds1". Crop Science 10 (1): 87–90. 1970. doi:10.2135/cropsci1970.0011183X001000010032x.
- ↑ 7.0 7.1 "Rapid defense responses in maize leaves induced by Spodoptera exigua caterpillar feeding". Journal of Experimental Botany 68 (16): 4709–4723. July 2017. doi:10.1093/jxb/erx274. PMID 28981781.
- ↑ "Additive effects of two quantitative trait loci that confer Rhopalosiphum maidis (corn leaf aphid) resistance in maize inbred line Mo17". Journal of Experimental Botany 66 (2): 571–8. February 2015. doi:10.1093/jxb/eru379. PMID 25249072.
- ↑ "Natural variation in maize defense against insect herbivores". Cold Spring Harbor Symposia on Quantitative Biology 77: 269–83. 2012. doi:10.1101/sqb.2012.77.014662. PMID 23223408.
- ↑ Jackson, Dave (2009). "Vegetative Shoot Meristems". in Bennetzen, Jeff L.; Hake, Sarah C.. Handbook of Maize: Its Biology. Springer New York. pp. 1–12. doi:10.1007/978-0-387-79418-1_1. ISBN 9780387794174. https://archive.org/details/handbookmaizeits00benn.
- ↑ 11.0 11.1 "Genetic variation at bx1 controls DIMBOA content in maize". Theoretical and Applied Genetics 120 (4): 721–34. February 2010. doi:10.1007/s00122-009-1192-1. PMID 19911162.
- ↑ 12.0 12.1 12.2 "Natural variation in maize aphid resistance is associated with 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside methyltransferase activity". The Plant Cell 25 (6): 2341–55. June 2013. doi:10.1105/tpc.113.112409. PMID 23898034.
- ↑ "Variation of DIMBOA and related compounds content in relation to the age and plant organ in maize". Phytochemistry 53 (2): 223–9. January 2000. doi:10.1016/S0031-9422(99)00498-7. PMID 10680175.
- ↑ "Prolonged expression of the BX1 signature enzyme is associated with a recombination hotspot in the benzoxazinoid gene cluster in Zea mays". Journal of Experimental Botany 66 (13): 3917–30. July 2015. doi:10.1093/jxb/erv192. PMID 25969552.
- ↑ "Highly localized and persistent induction of Bx1-dependent herbivore resistance factors in maize". The Plant Journal 88 (6): 976–991. December 2016. doi:10.1111/tpj.13308. PMID 27538820.
- ↑ "Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize". Plant Physiology 157 (1): 317–27. September 2011. doi:10.1104/pp.111.180224. PMID 21730199.
- ↑ 17.0 17.1 Hu, L.; Mateo, P.; Ye, M.; Zhang, X.; Berset, J. D.; Handrick, V.; Radisch, D.; Grabe, V. et al. (2018-08-17). "Plant iron acquisition strategy exploited by an insect herbivore" (in en). Science 361 (6403): 694–697. doi:10.1126/science.aat4082. ISSN 0036-8075. PMID 30115808. Bibcode: 2018Sci...361..694H.
 |

