Chemistry:Nickel phosphate
From HandWiki
Revision as of 00:18, 30 November 2020 by imported>TextAI2 (simplify)

| |
| Names | |
|---|---|
| IUPAC name
Nickel(2+) diphosphate
| |
| Other names
Nickel(II) phosphate, nickel diphosphate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Ni3(PO4)2 | |
| Molar mass | 366.022924 g/mol |
| Structure[1] | |
| Monoclinic, mP26 | |
| P21/c, No. 14 | |
a = 0.58273 nm, b = 0.46964 nm, c = 1.01059 nm α = 90°, β = 91.138°, γ = 90°
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Nickel phosphate is an inorganic compound with the formula Ni3(PO4)2. Its octahydrate Ni3(PO4)2·8(H2O) is a light green solid[2] that occurs as the mineral arupite.
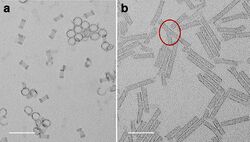
Ni phosphate nanorings and nanotubes. Scale bar 50 nm.[3]
References
- ↑ McMurdie, Howard F.; Morris, Marlene C.; Evans, Eloise H.; Paretzkin, Boris; Wong-Ng, Winnie; Zhang, Yuming; Hubbard, Camden R. (2013). "Standard X-Ray Diffraction Powder Patterns from the JCPDS Research Associateship". Powder Diffraction 2: 41–52. doi:10.1017/S0885715600012239.
- ↑ Perry, Dale L. (18 May 2011). Handbook of Inorganic Compounds, Second Edition. CRC Press. p. 292. ISBN 978-1-4398-1462-8. https://books.google.com/books?id=SFD30BvPBhoC&pg=PA292.
- ↑ Ni, Bing; Liu, Huiling; Wang, Peng-Peng; He, Jie; Wang, Xun (2015). "General synthesis of inorganic single-walled nanotubes". Nature Communications 6: 8756. doi:10.1038/ncomms9756. PMID 26510862.

