Chemistry:Nickel(II) hydroxide

| |
| |
| Names | |
|---|---|
| IUPAC name
Nickel(II) hydroxide
| |
| Other names
Nickel hydroxide, Theophrastite
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| Ni(OH)2 | |
| Molar mass | 92.724 g/mol (anhydrous) 110.72 g/mol (monohydrate) |
| Appearance | green crystals |
| Density | 4.10 g/cm3 |
| Melting point | 230 °C (446 °F; 503 K) (anhydrous, decomposes) |
| 0.0015 g/L[1] | |
Solubility product (Ksp)
|
5.48×10−16[2] |
| +4500.0·10−6 cm3/mol | |
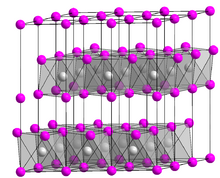
| Structure[3] | |
| hexagonal, hP3 | |
| P3m1, No. 164 | |
a = 0.3117 nm, b = 0.3117 nm, c = 0.4595 nm α = 90°, β = 90°, γ = 120°
| |
| Thermochemistry | |
Std molar
entropy (S |
79 J·mol−1·K−1[4] |
Std enthalpy of
formation (ΔfH⦵298) |
−538 kJ·mol−1[4] |
| Hazards | |
| Safety data sheet | External SDS |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H302, H332, H315, H334, H317, H341, H350, H360, H372 | |
| P260, P284, P201, P280, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1515 mg/kg (oral, rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nickel(II) hydroxide is the inorganic compound with the formula Ni(OH)2. It is a lime-green solid that dissolves with decomposition in ammonia and amines and is attacked by acids. It is electroactive, being converted to the Ni(III) oxy-hydroxide, leading to widespread applications in rechargeable batteries.[6]
Properties
Nickel(II) hydroxide has two well-characterized polymorphs, α and β. The α structure consists of Ni(OH)2 layers with intercalated anions or water.[7][8] The β form adopts a hexagonal close-packed structure of Ni2+ and OH− ions.[7][8] In the presence of water, the α polymorph typically recrystallizes to the β form.[7][9] In addition to the α and β polymorphs, several γ nickel hydroxides have been described, distinguished by crystal structures with much larger inter-sheet distances.[7]
The mineral form of Ni(OH)2, theophrastite, was first identified in the Vermion region of northern Greece, in 1980. It is found naturally as a translucent emerald-green crystal formed in thin sheets near the boundaries of idocrase or chlorite crystals.[10] A nickel-magnesium variant of the mineral, (Ni,Mg)(OH)
2 had been previously discovered at Hagdale on the island of Unst in Scotland.[11]
Reactions
Nickel(II) hydroxide is frequently used in electrical car batteries.[8] Specifically, Ni(OH)2 readily oxidizes to nickel oxyhydroxide, NiOOH, in combination with a reduction reaction, often of a metal hydride (reaction 1 and 2).[12][13]
Reaction 1
Ni(OH)
2 + OH− → NiO(OH) + H
2O + e−
Reaction 2
M + H
2O + e− → MH + OH−
Net Reaction (in H2O)
Ni(OH)
2 + M → NiOOH + MH
Of the two polymorphs, α-Ni(OH)2 has a higher theoretical capacity and thus is generally considered to be preferable in electrochemical applications. However, it transforms to β-Ni(OH)2 in alkaline solutions, leading to many investigations into the possibility of stabilized α-Ni(OH)2 electrodes for industrial applications.[9]
Synthesis
The synthesis entails treating aqueous solutions of nickel(II) salts with potassium hydroxide.[14]
Toxicity
The Ni2+ ion is a known carcinogen. Toxicity and related safety concerns have driven research into increasing the energy density of Ni(OH)2 electrodes, such as the addition of calcium or cobalt hydroxides.[6]
See also
- List of minerals named after people
- Nickel–cadmium battery
- Nickel–hydrogen battery
- Nickel–metal hydride battery
- Nickel–iron battery
References
- ↑ CRC Handbook of Chemistry and Physics (84 ed.). CRC press. 2003. pp. 4–71. ISBN 0849304849.
- ↑ John Rumble (June 18, 2018) (in English). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–189. ISBN 978-1138561632.
- ↑ Enoki, Toshiaki; Tsujikawa, Ikuji (1975). "Magnetic Behaviours of a Random Magnet, NipMg(1-p)(OH2)". Journal of the Physical Society of Japan 39 (2): 317. doi:10.1143/JPSJ.39.317. Bibcode: 1975JPSJ...39..317E.
- ↑ 4.0 4.1 Zumdahl, Steven S. (2009). Chemical Principles (6 ed.). Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ↑ "Nickel Hydroxide". American Elements. https://www.americanelements.com/nickel-hydroxide-12054-48-7.
- ↑ 6.0 6.1 Chen, J.; Bradhurst, D.H.; Dou, S.X.; Liu, H.K. (1999). "Nickel Hydroxide as an Active Material for the Positive Electrode in Rechargeable Alkaline Batteries". Journal of the Electrochemical Society 146 (10): 3606–3612. doi:10.1149/1.1392522. Bibcode: 1999JElS..146.3606C. https://ro.uow.edu.au/cgi/viewcontent.cgi?article=1118&context=engpapers.
- ↑ 7.0 7.1 7.2 7.3 Oliva, P.; Leonardi, J.; Laurent, J.F. (1982). "Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides". Journal of Power Sources 8 (2): 229–255. doi:10.1016/0378-7753(82)80057-8. Bibcode: 1982JPS.....8..229O.
- ↑ 8.0 8.1 8.2 Jeevanandam, P.; Koltypin, Y.; Gedanken, A. (2001). "Synthesis of Nanosized α-Nickel Hydroxide by a Sonochemical Method". Nano Letters 1 (5): 263–266. doi:10.1021/nl010003p. Bibcode: 2001NanoL...1..263J.
- ↑ 9.0 9.1 Shukla, A.K.; Kumar, V.G.; Munichandriah, N. (1994). "Stabilized α-Ni(OH)2 as Electrode Material for Alkaline Secondary Cells". Journal of the Electrochemical Society 141 (11): 2956–2959. doi:10.1149/1.2059264. Bibcode: 1994JElS..141.2956V.
- ↑ Marcopoulos, T.; Economou, M. (1980). "Theophrastite, Ni(OH)2, a new mineral from northern Greece". American Mineralogist 66: 1020–1021. http://www.minsocam.org/ammin/AM66/AM66_1020.pdf.
- ↑ Livingston, A.; Bish, D. L. (1982). "On the new mineral theophrastite, a nickel hydroxide, from Unst, Shetland, Scotland". Mineralogical Magazine 46 (338): 1. doi:10.1180/minmag.1982.046.338.01. Bibcode: 1982MinM...46....1L. http://www.minersoc.org/pages/Archive-MM/Volume_46/46-338-1.pdf.
- ↑ Ovshinsky, S.R.; Fetcenko, M.A.; Ross, J. (1993). "A nickel metal hydride battery for electric vehicles". Science 260 (5105): 176–181. doi:10.1126/science.260.5105.176. PMID 17807176. Bibcode: 1993Sci...260..176O.
- ↑ Young, Kwo (2016) (in en). Nickel Metal Hydride Batteries. MDPI. doi:10.3390/books978-3-03842-303-4. ISBN 978-3-03842-303-4. https://www.mdpi.com/books/pdfview/book/244.
- ↑ Glemser, O. (1963) "Nickel(II) Hydroxide" in ""Handbook of Preparative Inorganic Chemistry, 2nd ed. G. Brauer (ed.), Academic Press, NY. Vol. 1. p. 1549.
 |
