Biology:BH3 interacting-domain death agonist
 Generic protein structure example |
| BID | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 human pro-apoptotic protein bid | |||||||||
| Identifiers | |||||||||
| Symbol | BID | ||||||||
| Pfam | PF06393 | ||||||||
| InterPro | IPR010479 | ||||||||
| SCOP2 | 1ddb / SCOPe / SUPFAM | ||||||||
| TCDB | 1.A.21 | ||||||||
| OPM superfamily | 40 | ||||||||
| OPM protein | 2m5i | ||||||||
| |||||||||
The BH3 interacting-domain death agonist, or BID, gene is a pro-apoptotic member of the Bcl-2 protein family.[1] Bcl-2 family members share one or more of the four characteristic domains of homology entitled the Bcl-2 homology (BH) domains (named BH1, BH2, BH3 and BH4), and can form hetero- or homodimers. Bcl-2 proteins act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities.
Interactions
BID is a pro-apoptotic Bcl-2 protein containing only the BH3 domain. In response to apoptotic signaling, BID interacts with another Bcl-2 family protein, Bax, leading to the insertion of Bax into organelle membranes, primarily the outer mitochondrial membrane. Bax is believed to interact with, and induce the opening of the mitochondrial voltage-dependent anion channel, VDAC. Alternatively, growing evidence suggest that activated Bax and/or Bak form an oligomeric pore, MAC in the outer membrane. This results in the release of cytochrome c and other pro-apoptotic factors (such as SMAC/DIABLO)[2] from the mitochondria, often referred to as mitochondrial outer membrane permeabilization, leading to activation of caspases. This defines BID as a direct activator of Bax, a role common to some of the pro-apoptotic Bcl-2 proteins containing only the BH3 domain.
The anti-apoptotic Bcl-2 proteins, including Bcl-2 itself, can bind BID and inhibit BID's ability to activate Bax. As a result, the anti-apoptotic Bcl-2 proteins may inhibit apoptosis by sequestering BID, leading to reduced Bax activation.
The expression of BID is upregulated by the tumor suppressor p53, and BID has been shown to be involved in p53-mediated apoptosis.[3] The p53 protein is a transcription factor that, when activated as part of the cell's response to stress, regulates many downstream target genes, including BID. However, p53 also has a transcription-independent role in apoptosis. In particular, p53 interacts with Bax, promoting Bax activation and the insertion of Bax into the mitochondrial membrane.
The BH3 interacting-domain death agonist has been shown to interact with:
Cleavage
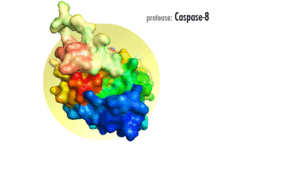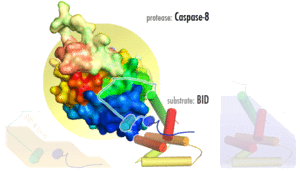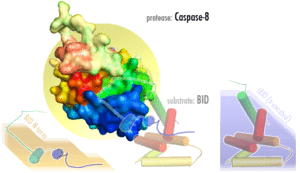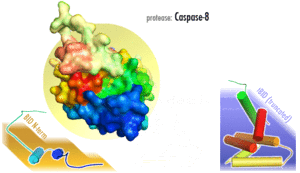
Several reports have demonstrated that caspase-8, and its substrate BID, are frequently activated in response to certain apoptotic stimuli in a death receptor-independent manner. N-hydroxy-L-arginine (NOHA), a stable intermediate product formed during the conversion of L-arginine to nitric oxide activates caspase-8.[12] Activation of caspase-8, and subsequent BID cleavage participate in cytochrome-c mediated apoptosis.[13] 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mediated activation of caspase-9 via cytochrome-c release has been shown to result in the activation of caspase-8 and Bid cleavage.[14] Aspirin and Curcumin (diferuloylmethane) too activate caspase-8 to cleave and translocate Bid, induced a conformational change in and translocation of Bax and cytochrome-c release.[15][16]
See also
References
- ↑ "BID: a novel BH3 domain-only death agonist". Genes Dev. 10 (22): 2859–69. 1996. doi:10.1101/gad.10.22.2859. PMID 8918887.
- ↑ Weinberg, Robert A. (2007). The biology of cancer. New York: Taylor & Francis. pp. 341. ISBN 978-0-8153-4076-8.
- ↑ "BID regulation by p53 contributes to chemosensitivity". Nat. Cell Biol. 4 (11): 842–9. 2002. doi:10.1038/ncb866. PMID 12402042.
- ↑ "Proapoptotic Bid mediates the Atr-directed DNA damage response to replicative stress". Cell Death Differ. 18 (5): 841–52. 2011. doi:10.1038/cdd.2010.151. PMID 21113148.
- ↑ 5.0 5.1 "Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function". Mol. Cell 17 (3): 393–403. 2005. doi:10.1016/j.molcel.2004.12.030. PMID 15694340.
- ↑ "Breast cancer cells can evade apoptosis-mediated selective killing by a novel small molecule inhibitor of Bcl-2". Cancer Res. 64 (21): 7947–53. 2004. doi:10.1158/0008-5472.CAN-04-0945. PMID 15520201.
- ↑ 7.0 7.1 "Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria". J. Biol. Chem. 277 (16): 13430–7. 2002. doi:10.1074/jbc.M108029200. PMID 11832478.
- ↑ "Caspase-2-induced apoptosis is dependent on caspase-9, but its processing during UV- or tumor necrosis factor-dependent cell death requires caspase-3". J. Biol. Chem. 276 (24): 21907–15. 2001. doi:10.1074/jbc.M011565200. PMID 11399776.
- ↑ "Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy". J. Biol. Chem. 280 (12): 11641–7. 2005. doi:10.1074/jbc.M411781200. PMID 15659383.
- ↑ "Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells". J. Biol. Chem. 280 (11): 10491–500. 2005. doi:10.1074/jbc.M412819200. PMID 15637055.
- ↑ "BID binds to replication protein A and stimulates ATR function following replicative stress". Mol. Cell. Biol. 31 (21): 4298–309. 2011. doi:10.1128/MCB.05737-11. PMID 21859891.
- ↑ "Caspase-8-mediated BID cleavage and release of mitochondrial cytochrome c during Nomega-hydroxy-L-arginine-induced apoptosis in MDA-MB-468 cells. Antagonistic effects of L-ornithine". J. Biol. Chem. 277 (40): 37630–6. 2002. doi:10.1074/jbc.M203648200. PMID 12145284.
- ↑ "Caspase-8 activation and bid cleavage contribute to MCF7 cellular execution in a caspase-3-dependent manner during staurosporine-mediated apoptosis". J. Biol. Chem. 275 (13): 9303–7. 2000. doi:10.1074/jbc.275.13.9303. PMID 10734071.
- ↑ "Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease". J. Neurosci. 21 (24): 9519–28. 2001. doi:10.1523/JNEUROSCI.21-24-09519.2001. PMID 11739563.
- ↑ "Activation of the caspase-8/Bid and Bax pathways in aspirin-induced apoptosis in gastric cancer". Carcinogenesis 26 (3): 541–6. 2005. doi:10.1093/carcin/bgh345. PMID 15579484.
- ↑ "Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl". Carcinogenesis 23 (1): 143–50. 2002. doi:10.1093/carcin/23.1.143. PMID 11756235.
External links
- Human BID genome location and BID gene details page in the UCSC Genome Browser.
 |

