Biology:Calprotectin
Calprotectin is a complex of the mammalian proteins S100A8 and S100A9.[1][2] Other names for calprotectin include MRP8-MRP14, calgranulin A and B, cystic fibrosis antigen, L1, 60BB antigen, and 27E10 antigen.[2][3] The proteins exist as homodimers but preferentially exist as S100A8/A9 heterodimers or heterotetramers (calprotectin) with antimicrobial, proinflammatory and prothrombotic properties.[4][5] In the presence of calcium, calprotectin is capable of sequestering the transition metals iron,[6] manganese and zinc[2][7] via chelation.[8] This metal sequestration affords the complex antimicrobial properties.[2][7] Calprotectin is the only known antimicrobial manganese sequestration protein complex.[9] Calprotectin comprises as much as 60% of the soluble protein content of the cytosol of a neutrophil,[2][10][11] and it is secreted by an unknown mechanism during inflammation.[3] Faecal calprotectin has been used to detect intestinal inflammation (colitis or enteritis) and can serve as a biomarker for inflammatory bowel diseases.[10][12] Blood-based calprotectin (in serum and plasma) is used in diagnostics of multiple inflammatory diseases, including autoimmune diseases, like arthritis, and severe infections including sepsis.[13][14]
Structure

The human homologue of calprotectin is a 24 kDa dimer,[9] and is formed by the protein monomers S100A8 (10,835 Da) and S100A9 (13,242 Da).[4][5] The primary structure of calprotectin can vary between species. For instance, the mouse homologue of S100A8 is 10,295 Da,[15] while the S100A9 homologue is 13,049 Da.[16] Early size exclusion chromatography experiments incorrectly indicated that calprotectin had a molecular mass of 36.5 kDa;[2][11] occasionally this value is used in contemporary literature. Calprotectin S100A8-S100A9 dimers can non-covalently pair with one another to form 48 kDa tetramers.
Metal binding
Calprotectin has a high affinity for calcium, zinc, iron, and manganese.[10][11][17][6] Each of S100A8 and S100A9 contain two EF-hand type Ca2+ binding sites,[9][3] and calprotectin is able to bind a total of four calcium ions per dimer or eight calcium ions per tetramer.[18] Calcium binding induces a conformational change in the complex that improves its affinity for transition metals, and promotes tetramer formation.[2][9] A maximum of two transition metal ions may bind to each calprotectin S100A8-S100A9 dimer.[9]
A calprotectin dimer can bind only one manganese or iron ion with high affinity, and it can do this only in the presence of calcium.[9][19][6] Zinc can bind at two sites within the calprotectin dimer, and this can occur in the absence of calcium.[2] Calcium, however, improves calprotectin's affinity for zinc.[9] While calprotectin metal binding occurs at the interface of S100A9 and S100A8 monomers, the independent monomers have some capacity for zinc binding, and may contribute to zinc homeostasis within mammals.[2][4][5]
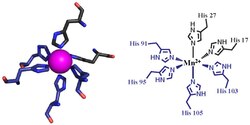
The first of the two calprotectin metal binding sites consists of a His3Asp motif, with S100A8 contributing two histidine ligands (His83 and His87), and S100A9 contributing a histidine and an aspartic acid ligand (His20 and Asp30).[9] The second site can coordinate metals through a tetra-histidine (His4) or a hexa-histidine (His6) binding motif. In the case of His4 binding, S100A8 coordinates through both His17 and His27 while S100A9 coordinates through His91 and His95.[9] In hexa-histidine binding two further histidine residues, His103 and His105, are recruited from the C-terminal end of S100A9 to enable octahedral coordination of the transition metal.[9] Manganese or iron are bound by the calprotectin dimer at this His6 site.[9][6] Zinc can be bound to either of the sites that form at the interface between S100A8 and S100A9 monomers.[9][19]
Inflammatory disease
Calprotectin constitutes up to 60% of soluble protein content in the cytosol of neutrophil granulocytes,[2][10][11] and it can be found at a lower concentration in monocytes, macrophages, and squamous epithelial cells.[2][10][11] Calprotectin enters into pus and abscess fluid during neutrophil cell death, along with other antimicrobial proteins.[2]
Mammalian cells secrete calprotectin during the inflammatory response. Circulating activated platelets and platelet-leukocyte aggregates are increased in acute and chronic sterile thrombo-inflammatory diseases. Plasma calprotectin is elevated in persons with metabolic syndrome, a disease characterized by chronic inflammation.[20] Calprotectin is secreted in the mouth during inflammation of the gingiva and during oral candidiasis infection.[21][22] People who have mutations in the calprotectin gene appear susceptible to serious gum infections.[21] Manganese sequestration by calprotectin is likely important during lung inflammation.[7] The exact mechanism by which S100A8 and S100A9 is secreted by mammalian cells during inflammation remains unknown.[3] In lung autopsies from patients with inflammation caused by COVID-19, heterodimeric S100A8/A9 is mainly detected in neutrophils and deposited on vessel walls.[23] Platelet glycoprotein Ib alpha (GP1BA;GPIbα) is the receptor for S100A8/A9 on platelets.[23] In vitro, platelets adhere to and partially spread on S100A8/A9, leading to the formation of distinct populations of P-selectin+ and phosphatidylserine+ platelets. The prothrombotic pathway initiated by interaction of S100A8/A9 with GPIbα induces the formation of procoagulant platelets and fibrin (CD36 has a supporting role).[23]
Antimicrobial properties
Transition metals are essential to the survival of all organisms.[24] Mammals strictly limit metal availability as a part of the innate immune system, and this helps prevent infection by microbes and fungi.[24] Calprotectin was first described in the 1980s as a mammalian antimicrobial protein that acts through the sequestration of zinc.[1][2][9] It is now known that calprotectin also has antibacterial and antifungal properties that arise from its ability to sequester manganese and iron.[7][9][6] Calprotectin is the only known antimicrobial agent that acts through manganese sequestration.[9]
Faecal calprotectin
Calprotectin becomes available in the intestinal lumen via leukocyte shedding,[1] active secretion,[2][11] cell disturbance, and cell death.[1][11] This results in elevated faecal calprotectin levels, which can be detected in the stool.[1][11] Elevated faecal calprotectin levels therefore indicate migration of neutrophils into the intestinal mucosa, which occurs during intestinal inflammation.[1][11][17] As people with active inflammatory bowel diseases (IBD) such as ulcerative colitis or Crohn disease have as much as a 10-fold increase in faecal calprotectin levels,[10] the measurement of faecal calprotectin can serve as a biochemical test for these diseases.
Although a relatively new test, faecal calprotectin is regularly used as an indicator for IBD during treatment and as a diagnostic marker.[12] Faecal calprotectin tests can also function in distinguishing patients with irritable bowel syndrome from those with IBD.[1][11] Calprotectin is useful as a marker, as it is resistant to enzymatic degradation, and can be easily measured in faeces.[25] Although faecal calprotectin correlates significantly with disease activity in people with confirmed IBD,[26] elevated faecal calprotectin can be a false-positive indicator of IBD under some conditions. Importantly, intake of proton pump inhibitor is associated with significantly elevated calprotectin values.[27] Furthermore, positive faecal calprotectin does not help in localizing IBD, or in distinguishing ulcerative colitis from Crohn's disease.[1] Faecal calprotectin can also indicate other gastrointestinal conditions such as colorectal cancer, gastroenteritis, and food intolerance.[1] Calprotectin levels vary depending on age, comorbidity, and may vary day-to-day within individuals.[1] Faecal calprotectin could be used as a preliminary screen in otherwise functional patients suspected of having IBD, or as a means of following mucosal healing.[1] In patients with SARS-CoV-2 infection, elevated faecal calprotectin has been demonstrated to correlated with COVID-19 induced thrombosis even in patients without gastrointestinal symptoms.[28] The potential for using faecal calprotectin in this way is debated, however, and cut-off levels have not been agreed upon.[1]
See also
- Zinc in biology
- Manganese in biology
- Nutritional role of calcium
- Bioinorganic Chemistry
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 "The role and utility of faecal markers in inflammatory bowel disease". Therapeutic Advances in Gastroenterology 8 (1): 23–36. January 2015. doi:10.1177/1756283X14553384. PMID 25553077.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 "Calprotectin - a pleiotropic molecule in acute and chronic inflammation". Physiological Research 53 (3): 245–53. 2004. doi:10.33549/physiolres.930448. PMID 15209531.
- ↑ 3.0 3.1 3.2 3.3 Celio, Marco R.; Pauls, Thomas; Schwaller, Beat (1996). Guidebook to the calcium-binding proteins. Oxford: Sambrook & Tooze Publication at Oxford University Press. pp. 147–148. ISBN 0198599501.
- ↑ 4.0 4.1 4.2 UniProt Consortium. "P05109- S10A8_HUMAN". UniProt Consortium. https://www.uniprot.org/uniprot/P05109.
- ↑ 5.0 5.1 5.2 UniProt Consortium. "P06702- S10A9_HUMAN". UniProt Consortium. https://www.uniprot.org/uniprot/P06702.
- ↑ 6.0 6.1 6.2 6.3 6.4 "Human calprotectin is an iron-sequestering host-defense protein". Nature Chemical Biology 11 (10): 765–71. October 2015. doi:10.1038/nchembio.1891. PMID 26302479.
- ↑ 7.0 7.1 7.2 7.3 Costa, Lucio G; Aschner, Michael (2014). Manganese in Health and Disease. Royal Society of Chemistry. p. 146. ISBN 978-1849739436. https://books.google.com/books?id=3UG9BQAAQBAJ. Retrieved 27 January 2015.
- ↑ Clark, HL (2016), "Zinc and manganese chelation by neutrophil s100a8/a9 (calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection", J Immunol 196 (1): 336–344, doi:10.4049/jimmunol.1502037, PMID 26582948.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 9.13 9.14 "Manganese and microbial pathogenesis: sequestration by the Mammalian immune system and utilization by microorganisms". ACS Chemical Biology 10 (3): 641–51. March 2015. doi:10.1021/cb500792b. PMID 25594606.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Marshall, William Marshall; Lapsley, Marta; Day, Andrew; Ayling, Ruth (2014). Clinical Biochemistry: Metabolic and Clinical Aspects (3 ed.). Elsevier Health Sciences, 2014. ISBN 9780702054785. https://books.google.com/books?id=2FkXAwAAQBAJ&dq=faecal+calprotectin&pg=PT529. Retrieved 19 January 2015.
- ↑ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 Gupta, Ramesh (2014). Biomarkers in toxicology. San Diego, CA: Academic Press. pp. 272–273. ISBN 9780124046498. https://books.google.com/books?id=EMpUAgAAQBAJ. Retrieved 19 January 2015.
- ↑ 12.0 12.1 "Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis". BMJ 341: c3369. July 2010. doi:10.1136/bmj.c3369. PMID 20634346.
- Fran Lowry (July 22, 2010). "Fecal Calprotectin a Sign of Inflammatory Bowel Disease". http://www.medscape.com/viewarticle/725672.
- ↑ Pruenster, Monika; Vogl, Thomas; Roth, Johannes; Sperandio, Markus (November 2016). "S100A8/A9: From basic science to clinical application". Pharmacology & Therapeutics 167: 120–131. doi:10.1016/j.pharmthera.2016.07.015. ISSN 1879-016X. PMID 27492899. https://pubmed.ncbi.nlm.nih.gov/27492899.
- ↑ Chan, James K.; Roth, Johannes; Oppenheim, Joost J.; Tracey, Kevin J.; Vogl, Thomas; Feldmann, Marc; Horwood, Nicole; Nanchahal, Jagdeep (August 2012). "Alarmins: awaiting a clinical response". The Journal of Clinical Investigation 122 (8): 2711–2719. doi:10.1172/JCI62423. ISSN 1558-8238. PMID 22850880.
- ↑ UniProt Consortium. "P27005- S10A8_MOUSE". UniProt Consortium. https://www.uniprot.org/uniprot/P27005.
- ↑ UniProt Consortium. "P31725- S10A9_MOUSE". UniProt Consortium. https://www.uniprot.org/uniprot/P31725.
- ↑ 17.0 17.1 Evans, G.O. (2009). Animal Clinical Chemistry: A Practical Handbook for Toxicologists and Biomedical Researchers (2 ed.). Boca Raton: Taylor & Francis. pp. 107–108. ISBN 9781420080124. https://books.google.com/books?id=lIIDiaGfcrkC. Retrieved 19 January 2015.
- ↑ "Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 are confirmed by electrospray ionization-mass analysis". Journal of the American Society for Mass Spectrometry 11 (9): 780–8. September 2000. doi:10.1016/s1044-0305(00)00150-1. PMID 10976885.
- ↑ 19.0 19.1 Maret, Wolfgang; Wedd, Anthony (2014). Binding, transport and storage of metal ions in biological cells. [S.l.]: Royal Soc Of Chemistry. p. 271. ISBN 9781849735995. https://books.google.com/books?id=8ggdBAAAQBAJ. Retrieved 27 January 2015.
- ↑ "Plasma calprotectin and its association with cardiovascular disease manifestations, obesity and the metabolic syndrome in type 2 diabetes mellitus patients". BMC Cardiovascular Disorders 14: 196. December 2014. doi:10.1186/1471-2261-14-196. PMID 25527236.
- ↑ 21.0 21.1 Schaechter, Moselio (2009). Encyclopedia of microbiology (3 ed.). [S.l.]: Elsevier. p. 570. ISBN 978-0123739445. https://books.google.com/books?id=rLhdW5YzuO4C. Retrieved 27 January 2015.
- ↑ Vacharaksa, Anjalee (2007). Restricted HIV-1 Infection Increases Susceptibility of Candida Infection in Oral Keratinocytes. p. 20. ISBN 9780549367666. https://books.google.com/books?id=Ces7swEMogwC. Retrieved 27 January 2015.
- ↑ 23.0 23.1 23.2 "S100A8/A9 drives the formation of procoagulant platelets through GPIbα". Blood 2022 (24): 2626–2643. August 26, 2022. doi:10.1182/blood.2021014966. PMID 36026606.
- ↑ 24.0 24.1 "Nutritional immunity: transition metals at the pathogen-host interface". Nature Reviews. Microbiology 10 (8): 525–37. July 2012. doi:10.1038/nrmicro2836. PMID 22796883.
- ↑ "A simple method for assessing intestinal inflammation in Crohn's disease". Gut 47 (4): 506–13. October 2000. doi:10.1136/gut.47.4.506. PMID 10986210.
- ↑ "Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease". Inflammatory Bowel Diseases 18 (12): 2218–24. December 2012. doi:10.1002/ibd.22917. PMID 22344983.
- ↑ "Proton pump inhibitors are associated with elevation of faecal calprotectin and may affect specificity". European Journal of Gastroenterology & Hepatology 15 (5): 573–4; author reply 574. May 2003. doi:10.1097/00042737-200305000-00021. PMID 12702920.
- ↑ Giuffrè, Mauro; Di Bella, Stefano; Sambataro, Gianluca; Zerbato, Verena; Cavallaro, Marco; Occhipinti, Alessandro Agostino; Palermo, Andrea; Crescenzi, Anna et al. (2020-09-16). "COVID-19-Induced Thrombosis in Patients without Gastrointestinal Symptoms and Elevated Fecal Calprotectin: Hypothesis Regarding Mechanism of Intestinal Damage Associated with COVID-19" (in en). Tropical Medicine and Infectious Disease 5 (3): 147. doi:10.3390/tropicalmed5030147. ISSN 2414-6366. PMID 32947803.
 |
