Biology:Carboxylesterase type B
| Carboxylesterase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
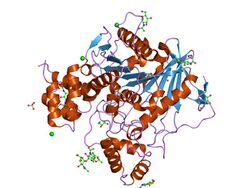 Structure of ethylphosphorylated Butyrylcholinesterase.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | COesterase | ||||||||
| Pfam | PF00135 | ||||||||
| InterPro | IPR002018 | ||||||||
| PROSITE | PDOC00112 | ||||||||
| SCOP2 | 1acj / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 127 | ||||||||
| OPM protein | 1p0i | ||||||||
| CDD | cd00312 | ||||||||
| |||||||||
Carboxylesterase, type B is a family of evolutionarily related proteins that belongs to the superfamily of proteins with the Alpha/beta hydrolase fold.
Higher eukaryotes have many distinct esterases. The different types include those that act on carboxylic esters (EC 3.1.1). Carboxyl-esterases have been classified into three categories (A, B and C) on the basis of differential patterns of inhibition by organophosphates. The sequence of a number of type-B carboxylesterases indicates[2][3][4] that the majority are evolutionarily related. As is the case for lipases and serine proteases, the catalytic apparatus of esterases involves three residues (catalytic triad): a serine, a glutamate or aspartate and a histidine.
Subfamilies
Examples
Human genes that encode proteins containing the carboxylesterase domain include:
See also
References
- ↑ "Role of water in aging of human butyrylcholinesterase inhibited by echothiophate: the crystal structure suggests two alternative mechanisms of aging". Biochemistry 44 (4): 1154–62. February 2005. doi:10.1021/bi048238d. PMID 15667209.
- ↑ "On the origins of esterases". Mol. Biol. Evol. 5 (2): 113–119. 1988. doi:10.1093/oxfordjournals.molbev.a040485. PMID 3163407.
- ↑ "Cholinesterase-like domains in enzymes and structural proteins: functional and evolutionary relationships and identification of a catalytically essential aspartic acid". Proc. Natl. Acad. Sci. U.S.A. 88 (15): 6647–6651. 1991. doi:10.1073/pnas.88.15.6647. PMID 1862088. Bibcode: 1991PNAS...88.6647K.
- ↑ "Relationship between sequence conservation and three-dimensional structure in a large family of esterases, lipases, and related proteins". Protein Sci. 2 (3): 366–382. 1993. doi:10.1002/pro.5560020309. PMID 8453375.
External links
 |

