Biology:Environmental DNA

Environmental DNA or eDNA is DNA that is collected from a variety of environmental samples such as soil, seawater, snow or air, rather than directly sampled from an individual organism. As various organisms interact with the environment, DNA is expelled and accumulates in their surroundings from various sources.[2]
In recent years, eDNA has been used as a tool to detect endangered wildlife that were otherwise unseen. In 2020, human health researchers began repurposing eDNA techniques to track the COVID-19 pandemic.[3]
Example sources of eDNA include, but are not limited to, feces, mucus, gametes, shed skin, carcasses and hair.[2][4] Samples can be analyzed by high-throughput DNA sequencing methods, known as metagenomics, metabarcoding, and single-species detection, for rapid monitoring and measurement of biodiversity. In order to better differentiate between organisms within a sample, DNA metabarcoding is used in which the sample is analyzed and uses previously studied DNA libraries, such as BLAST, to determine what organisms are present.[5]
eDNA metabarcoding is a novel method of assessing biodiversity wherein samples are taken from the environment via water, sediment or air from which DNA is extracted, and then amplified using general or universal primers in polymerase chain reaction and sequenced using next-generation sequencing to generate thousands to millions of reads. From this data, species presence can be determined, and overall biodiversity assessed. It is an interdisciplinary method that brings together traditional field-based ecology with in-depth molecular methods and advanced computational tools.[6]
The analysis of eDNA has great potential, not only for monitoring common species, but to genetically detect and identify other extant species that could influence conservation efforts.[7] This method allows for biomonitoring without requiring collection of the living organism, creating the ability to study organisms that are invasive, elusive, or endangered without introducing anthropogenic stress on the organism. Access to this genetic information makes a critical contribution to the understanding of population size, species distribution, and population dynamics for species not well documented. Importantly, eDNA is often more cost-effective compared to traditional sampling methods.[8] The integrity of eDNA samples is dependent upon its preservation within the environment.
Soil, permafrost, freshwater and seawater are well-studied macro environments from which eDNA samples have been extracted, each of which include many more conditioned subenvironments.[9] Because of its versatility, eDNA is applied in many subenvironments such as freshwater sampling, seawater sampling, terrestrial soil sampling (tundra permafrost), aquatic soil sampling (river, lake, pond, and ocean sediment),[10] or other environments where normal sampling procedures can become problematic.[9]
On 7 December 2022, The New York Times reported that two-million year old eDNA genetic material was found in Greenland, and is currently considered the oldest DNA discovered so far.[11][12]
Overview
Environmental DNA or eDNA describes the genetic material present in environmental samples such as sediment, water, and air, including whole cells, extracellular DNA and potentially whole organisms.[13][14] The analyse of eDNA start with capturing an environmental sample of interest. The DNA in the sample is extracted and purified. The purified DNA is then amplified for a specific gene target so it can be sequenced and categorised based on its sequence.[15] From this information, detection and classification of species is possible.[6]
eDNA can come from skin, mucous, saliva, sperm, secretions, eggs, feces, urine, blood, roots, leaves, fruit, pollen, and rotting bodies of larger organisms, while microorganisms may be obtained in their entirety.[16][7][14] eDNA production is dependent on biomass, age and feeding activity of the organism as well as physiology, life history, and space use.[2][17][14][18][19][6]
Despite being a relatively new method of surveying, eDNA has already proven to have enormous potential in biological monitoring. Conventional methods for surveying richness and abundance are limited by taxonomic identification, may cause disturbance or destruction of habitat, and may rely on methods in which it is difficult to detect small or elusive species, thus making estimates for entire communities impossible. eDNA can complement these methods by targeting different species, sampling greater diversity, and increasing taxonomic resolution.[20] Additionally, eDNA is capable of detecting rare species,[21][17] but not of determining population quality information such as sex ratios and body conditions, so it is ideal for supplementing traditional studies.[18][20] Regardless, it has useful applications in detecting the first occurrences of invasive species, the continued presence of native species thought to be extinct or otherwise threatened, and other elusive species occurring in low densities that would be difficult to detect by traditional means.[6]
Degradation of eDNA in the environment limits the scope of eDNA studies, as often only small segments of genetic material remain, particularly in warm, tropical regions. Additionally, the varying lengths of time to degradation based on environmental conditions and the potential of DNA to travel throughout media such as water can affect inference of fine-scale spatiotemporal trends of species and communities.[17][22][16][23][18][20][19] Despite these drawbacks, eDNA still has the potential to determine relative or rank abundance as some studies have found it to correspond with biomass, though the variation inherent in environmental samples makes it difficult to quantify.[7][14] While eDNA has numerous applications in conservation, monitoring, and ecosystem assessment, as well as others yet to be described, the highly variable concentrations of eDNA and potential heterogeneity through the water body makes it essential that the procedure is optimized, ideally with a pilot study for each new application to ensure that the sampling design is appropriate to detect the target.[24][18][20][6]
Community DNA
While the definition of eDNA seems straightforward, the lines between different forms of DNA become blurred, particularly in comparison to community DNA, which is described as bulk organismal samples.[20] A question arises regarding whole microorganisms captured in eDNA samples: do these organisms alter the classification of the sample to a community DNA sample? Additionally, the classification of genetic material from feces is problematic and often referred to as eDNA.[20] Differentiation between the two is important as community DNA indicates organismal presence at a particular time and place, while eDNA may have come from a different location, from predator feces, or from past presence, however this differentiation is often impossible.[25][20] However, eDNA can be loosely classified as including many sectors of DNA biodiversity research, including fecal analysis and bulk samples when they are applicable to biodiversity research and ecosystem analysis.[6]
selfDNA
The concept of selfDNA stems from discoveries made by scientists from the University of Naples Federico II, which were reported during 2015 in the journal New Phytologist,[26] about the self-inhibitory effect of extracellular DNA in plants,[27] but also in bacteria, fungi, algae, plants, protozoa and insects.[28] The environmental source of such extracellular DNA is proposed to be plant litter but also other sources in different ecosystems and organisms, with the size of DNA fragments experimentally shown to have an inhibitory effect upon their conspecific organisms typically ranging between 200 and 500 base pairs. The selfDNA phenomenon has been postulated to drive ecological interactions and to be mechanistically mediated by damage-associated molecular patterns (DAMPs)[29][30] and to have potential for the development of biocidal applications.[31]
eDNA metabarcoding
-
Applications of environmental DNA metabarcoding in aquatic and terrestrial ecosystems [6]
By 2019 methods in eDNA research had been expanded to be able to assess whole communities from a single sample. This process involves metabarcoding, which can be precisely defined as the use of general or universal polymerase chain reaction (PCR) primers on mixed DNA samples from any origin followed by high-throughput next-generation sequencing (NGS) to determine the species composition of the sample. This method has been common in microbiology for years, but is only just finding its footing in assessment of macroorganisms.[32][22][25][20] Ecosystem-wide applications of eDNA metabarcoding have the potential to not only describe communities and biodiversity, but also to detect interactions and functional ecology over large spatial scales,[33] though it may be limited by false readings due to contamination or other errors.[32][7][34][25][19] Altogether, eDNA metabarcoding increases speed, accuracy, and identification over traditional barcoding and decreases cost, but needs to be standardized and unified, integrating taxonomy and molecular methods for full ecological study.[22][35][36][37][19][6][38]
eDNA metabarcoding has applications to diversity monitoring across all habitats and taxonomic groups, ancient ecosystem reconstruction, plant-pollinator interactions, diet analysis, invasive species detection, pollution responses, and air quality monitoring. eDNA metabarcoding is a unique method still in development and will likely remain in flux for some time as technology advances and procedures become standardized. However, as metabarcoding is optimized and its use becomes more widespread, it is likely to become an essential tool for ecological monitoring and global conservation study.[6]
Extracellular and relic DNA
-
Relic DNA dynamics [39]
Extracellular DNA, sometimes called relic DNA, is DNA from dead microbes. Naked extracellular DNA (eDNA), most of it released by cell death, is nearly ubiquitous in the environment. Its concentration in soil may be as high as 2 μg/L, and its concentration in natural aquatic environments may be as high at 88 μg/L.[40] Various possible functions have been proposed for eDNA: it may be involved in horizontal gene transfer;[41] it may provide nutrients;[42] and it may act as a buffer to recruit or titrate ions or antibiotics.[43] Extracellular DNA acts as a functional extracellular matrix component in the biofilms of several bacterial species. It may act as a recognition factor to regulate the attachment and dispersal of specific cell types in the biofilm;[44] it may contribute to biofilm formation;[45] and it may contribute to the biofilm's physical strength and resistance to biological stress.[46]
Under the name of environmental DNA, eDNA has seen increased use in the natural sciences as a survey tool for ecology, monitoring the movements and presence of species in water, air, or on land, and assessing an area's biodiversity.[47][48]
In the diagram on the right, the amount of relic DNA in a microbial environment is determined by inputs associated with the mortality of viable individuals with intact DNA and by losses associated with the degradation of relic DNA. If the diversity of sequences contained in the relic DNA pool is sufficiently different from that in the intact DNA pool, then relic DNA may bias estimates of microbial biodiversity (as indicated by different colored boxes) when sampling from the total (intact + relic) DNA pool.[39] Standardised Data on Initiatives (STARDIT) has been proposed as one way of standardising both data about sampling and analysis methods, and taxonomic and ontological relationships.[49]
Collection
Terrestrial sediments
- Methods in modern and ancient marine genomics
-
(a) Metabarcoding is the amplification and analysis of equally sized DNA fragments from a total DNA extract. (b) Metagenomics is the extraction, amplification, and analysis of all DNA fragments independent of size. (c) Target-capture describes the enrichment and analysis of specific (chosen) DNA fragments independent of size from a total DNA extract.[50]
The importance of eDNA analysis stemmed from the recognition of the limitations presented by culture-based studies.[7] Organisms have adapted to thrive in the specific conditions of their natural environments. Although scientists work to mimic these environments, many microbial organisms can not be removed and cultured in a laboratory setting.[9] The earliest version of this analysis began with ribosomal RNA (rRNA) in microbes to better understand microbes that live in hostile environments.[51] The genetic makeup of some microbes is then only accessible through eDNA analysis. Analytical techniques of eDNA were first applied to terrestrial sediments yielding DNA from both extinct and extant mammals, birds, insects and plants.[52] Samples extracted from these terrestrial sediments are commonly referenced as 'sedimentary ancient DNA' (sedaDNA or dirtDNA).[53] The eDNA analysis can also be used to study current forest communities including everything from birds and mammals to fungi and worms.[9] Samples can be obtained from soil, faeces, 'bite DNA' from where leaves have been bitten, plants and leaves where animals have been, and from the blood meals of captured mosquitos which may have eaten blood from any animals in the area.[54] Some methods can also attempt to capture cells with hair traps and sandpaper in areas commonly transversed by target species.
Aquatic sediments
The sedaDNA was subsequently used to study ancient animal diversity and verified using known fossil records in aquatic sediments.[9] The aquatic sediments are deprived of oxygen and are thus protect the DNA from degrading.[9] Other than ancient studies, this approach can be used to understand current animal diversity with relatively high sensitivity. While typical water samples can have the DNA degrade relatively quickly, the aquatic sediment samples can have useful DNA two months after the species was present.[55] One problem with aquatic sediments is that it is unknown where the organism deposited the eDNA as it could have moved in the water column.
-
Drilling vessel recovering a sediment core for sedaDNA analysis and hypothetical past marine community composition [50]
-
Subglacial aquatic sediment continuous coring [56]
Aquatic (water column)
Studying eDNA in the water column can indicate the community composition of a body of water. Before eDNA, the main ways to study open water diversity was to use fishing and trapping, which requires resources such as funding and skilled labour, whereas eDNA only needs samples of water.[10] This method is effective as pH of the water does not affect the DNA as much as previously thought, and sensitivity can be increased relatively easily.[10][57] Sensitivity is how likely the DNA marker will be present in the sampled water, and can be increased simply by taking more samples, having bigger samples, and increasing PCR.[57] eDNA degrades relatively fast in the water column, which is very beneficial in short term conservation studies such as identifying what species are present.[9]
Researchers at the Experimental Lakes Area in Ontario, Canada and McGill University have found that eDNA distribution reflects lake stratification.[58] As seasons and water temperature change, water density also changes such that it forms distinct layers in small boreal lakes in the summer and winter. These layers mix during the spring and fall.[59] Fish habitat use correlates to stratification (e.g. a cold-water fish like lake trout will stay in cold water) and so does eDNA distribution, as these researchers found.[58]
Monitoring species
eDNA can be used to monitor species throughout the year and can be very useful in conservation monitoring.[17][60][61] eDNA analysis has been successful at identifying many different taxa from aquatic plants,[62] aquatic mammals,[21][17] fishes,[32][61] mussels,[60] fungi [63][64] and even parasites.[65][51] eDNA has been used to study species while minimizing any stress inducing human interaction, allowing researchers to monitor species presence at larger spatial scales more efficiently.[66][67] The most prevalent use in current research is using eDNA to study the locations of species at risk, invasive species, and keystone species across all environments.[66] eDNA is especially useful for studying species with small populations because eDNA is sensitive enough to confirm the presence of a species with relatively little effort to collect data which can often be done with a soil sample or water sample.[7][66] eDNA relies on the efficiency of genomic sequencing and analysis as well as the survey methods used which continue to become more efficient and cheaper.[68] Some studies have shown that eDNA sampled from stream and inshore environment decayed to undetectable level at within about 48 hours.[69][70]
Environmental DNA can be applied as a tool to detect low abundance organisms in both active and passive forms. Active eDNA surveys target individual species or groups of taxa for detection by using highly sensitive species-specific quantitative real-time PCR[71] or digital droplet PCR markers.[72] CRISPR-Cas methodology has also been applied to the detection of single species from eDNA;[73] utilising the Cas12a enzyme and allowing greater specificity when detecting sympatric taxa. Passive eDNA surveys employ massively-parallel DNA sequencing to amplify all eDNA molecules in a sample with no a priori target in mind providing blanket DNA evidence of biotic community composition.[74]
Decline of terrestrial arthropods
- Differentiation of arthropod communities by plant species
-
Bipartite plot for the COI gene, showing from which plants each arthropod family is obtained. Plant names: Angeli (Angelica archangelica), Centau (Centaurea jacea), Daucus (Daucus carota), Echium (Echium vulgare), Eupato (Eupatorium cannabinum), Solida (Solidago canadensis), Tanace (Tanacetum vulgare).[1]
Terrestrial arthropods are experiencing massive decline in Europe as well as globally,[75][76][77][78] although only a fraction of the species have been assessed and the majority of insects are still undescribed to science.[79] As one example, grassland ecosystems are home to diverse taxonomic and functional groups of terrestrial arthropods, such as pollinators, phytophagous insects, and predators, that use nectar and pollen for food sources, and stem and leaf tissue for food and development. These communities harbor endangered species, since many habitats have disappeared or are under significant threat.[80][81] Therefore, extensive efforts are being conducted in order to restore European grassland ecosystems and conserve biodiversity.[82] For instance, pollinators like bees and butterflies represent an important ecological group that has undergone severe decline in Europe, indicating a dramatic loss of grassland biodiversity.[83][84][85][86] The vast majority of flowering plants are pollinated by insects and other animals both in temperate regions and the tropics.[87] The majority of insect species are herbivores feeding on different parts of plants, and most of these are specialists, relying on one or a few plant species as their main food resource.[88] However, given the gap in knowledge on existing insect species, and the fact that most species are still undescribed, it is clear that for the majority of plant species in the world, there is limited knowledge about the arthropod communities they harbor and interact with.[1]
Terrestrial arthropod communities have traditionally been collected and studied using methods, such as Malaise traps and pitfall traps, which are very effective but somewhat cumbersome and potentially invasive methods. In some instances, these techniques fall short of performing efficient and standardized surveys, due to, for example, phenotypic plasticity, closely related species, and difficulties in identifying juvenile stages. Furthermore, morphological identification depends directly on taxonomic expertise, which is in decline.[89][90][91] All such limitations of traditional biodiversity monitoring have created a demand for alternative approaches. Meanwhile, the advance in DNA sequencing technologies continuously provides new means of obtaining biological data.[7][92][25][9] Hence, several new molecular approaches have recently been suggested for obtaining fast and efficient data on arthropod communities and their interactions through non‐invasive genetic techniques. This includes extracting DNA from sources such as bulk samples or insect soups,[93][94][95][96] empty leaf mines,[97] spider webs,[98] pitcher plant fluid,[99] environmental samples like soil, water, air, and even whole flowers (environmental DNA [eDNA]),[100][101][102][9][103] host plant and predatory diet identification from insect DNA extracts,[104][105] and predator scat from bats.[106][107] Recently, also DNA from pollen attached to insects has been used for retrieving information on plant–pollinator interactions.[108][109] Many of such recent studies rely on DNA metabarcoding—high‐throughput sequencing of PCR amplicons using generic primers.[110][101][1]
Mammals
-
Canada lynx
-
Tracks of a Canada lynx in snow
Snow tracks
Wildlife researchers in snowy areas also use snow samples to gather and extract genetic information about species of interest. DNA from snow track samples has been used to confirm the presence of such elusive and rare species as polar bears, arctic fox, lynx, wolverines, and fishers.[111][112][113][114]
DNA from the air
In 2021, researchers demonstrated that eDNA can be collected from air and used to identify mammals.[115][116][117][118] In 2023, scientists developed a specialized sampling probe and aircraft surveys to assess biodiversity of multiple taxa, including mammals, using air eDNA.[119]
Managing fisheries
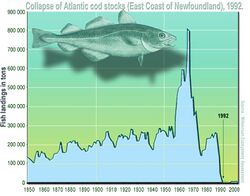

The successful management of commercial fisheries relies on standardised surveys to estimate the quantity and distribution of fish stocks. Atlantic cod (Gadus morhua) is an iconic example that demonstrates how poorly constrained data and uninformed decision making can result in catastrophic stock decline and ensuing economic and social problems.[121] Traditional stock assessments of demersal fish species have relied primarily on trawl surveys, which have provided a valuable stream of information to decision makers.[122] However, there are some notable drawbacks of demersal trawl surveys including cost,[123] gear selectivity/catchability,[124] habitat destruction[125] and restricted coverage (e.g. hard-substrate bottom environments, marine protected areas).[126]
Environmental DNA (eDNA) has emerged as a potentially powerful alternative for studying ecosystem dynamics. The constant loss and shedding of genetic material from macroorganisms imparts a molecular footprint in environmental samples that can be analysed to determine either the presence of specific target species [13][127] or characterise biodiversity.[128][129] The combination of next generation sequencing and eDNA sampling has been successfully applied in aquatic systems to document spatial and temporal patterns in the diversity of fish fauna.[130][131][132][133] To further develop the utility of eDNA for fisheries management, understanding the ability of eDNA quantities to reflect fish biomass in the ocean is an important next step.[126]
Positive relationships between eDNA quantities and fish biomass and abundance have been demonstrated in experimental systems.[134][135][136] However, known variations between eDNA production [137][138] and degradation [139][140][141][142] rates is anticipated to complicate these relationships in natural systems. Furthermore, in oceanic systems, large habitat volumes and strong currents are likely to result in physical dispersal of DNA fragments away from target organisms.[143] These confounding factors have been previously considered to restrict the application of quantitative eDNA monitoring in oceanic settings.[143][126]
Despite these potential constraints, numerous studies in marine environments have found positive relationships between eDNA quantities and complimentary survey efforts including radio-tagging,[144] visual surveys,[133][145] echo-sounding [146] and trawl surveys.[132][147] However, studies that quantify target eDNA concentrations of commercial fish species with standardised trawl surveys in marine environments are much scarcer.[147] In this context, direct comparisons of eDNA concentrations with biomass and stock assessment metrics, such as catch per unit effort (CPUE), are necessary to understand the applicability of eDNA monitoring to contribute to fisheries management efforts.[126]
Deep sea sediments
-
OTU (operational taxonomic unit) network of the extracellular DNA pools from the sediments of the different continental margins.[148]
Extracellular DNA in surface deep-sea sediments is by far the largest reservoir of DNA of the world oceans.[149] The main sources of extracellular DNA in such ecosystems are represented by in situ DNA release from dead benthic organisms, and/or other processes including cell lysis due to viral infection, cellular exudation and excretion from viable cells, virus decomposition, and allochthonous inputs from the water column.[149][150][151][152] Previous studies provided evidence that an important fraction of extracellular DNA can escape degradation processes, remaining preserved in the sediments.[153][154] This DNA represents, potentially, a genetic repository that records biological processes occurring over time.[155][156][148]
Recent investigations revealed that DNA preserved in marine sediments is characterized by a large number of highly diverse gene sequences.[155][156][157] In particular, extracellular DNA has been used to reconstruct past prokaryotic and eukaryotic diversity in benthic ecosystems characterized by low temperatures and/or permanently anoxic conditions.[157][158][159][160][161][148]
The diagram on the right shows the OTU (operational taxonomic unit) network of the extracellular DNA pools from the sediments of the different continental margins. The dot size within the network is proportional to the abundance of sequences for each OTU. Dots circled in red represent extracellular core OTUs, dot circled in yellow are partially shared (among two or more pools) OTUs, dots circled in black are OTUs exclusive of each pool. The core OTUs contributing at least for 20 sequences are shown. The numbers in parentheses represent the number of connections among OTUs and samples: 1 for exclusive OTUs, 2–3 for partially shared OTUs and 4 for core OTUs.[148]
Previous studies suggested that the preservation of DNA might be also favoured in benthic systems characterised by high organic matter inputs and sedimentation rates, such as continental margins,.[162][163] These systems, which represent ca. 15% of the global seafloor, are also hotspots of benthic prokaryotic diversity,[164][165][166] and therefore they could represent optimal sites to investigate the prokaryotic diversity preserved within extracellular DNA.[148]
Spatial distribution of prokaryotic diversity has been intensively studied in benthic deep-sea ecosystems [167][168][169][170] through the analysis of "environmental DNA" (i.e., the genetic material obtained directly from environmental samples without any obvious signs of biological source material).[9] However, the extent to which gene sequences contained within extracellular DNA can alter the estimates of the diversity of the present-day prokaryotic assemblages is unknown.[171][148]
Sedimentary ancient DNA
Analyses of ancient DNA preserved in various archives have transformed understanding of the evolution of species and ecosystems. Whilst earlier studies have concentrated on DNA extracted from taxonomically constrained samples (such as bones or frozen tissue), advances in high-throughput sequencing and bioinformatics now allow the analysis of ancient DNA extracted from sedimentary archives,[172] so called sedaDNA. The accumulation and preservation of sedaDNA buried in land and lake sediments have been subject to active research and interpretation.[173] However, studying the deposition of DNA on the ocean floor and its preservation in marine sediments is more complex because the DNA has to travel through a water column for several kilometers.[174] Unlike in the terrestrial environment, with pervasive transport of subfossil biomass from land, the largest portion of the marine sedaDNA is derived from planktonic community, which is dominated by marine microbes and marine protists.[175] After the death of the surface plankton, its DNA is subject to a transport through the water column, during which much of the associated organic matter is known to be consumed and respired.[176] This transport could take between 3 and 12 days depending on the size and morphology of test.[177] However, it remains unclear how exactly the planktonic eDNA, defined as the total DNA present in the environment after,[178] survives this transport, whether the degradation or transport are associated with sorting or lateral advection, and finally, whether the eDNA arriving at the seafloor is preserved in marine sediments without further distortion of its composition.[179]
Despite the long exposure to degradation under oxic conditions during transport in the water column, and substantially lower concentration of organic matter on the seafloor, there is evidence that planktonic eDNA is preserved in marine sediments and contains exploitable ecological signal.[180] Earlier studies have shown sedaDNA preservation in marine sediments deposited under anoxia with unusually high amounts of organic matter preserved,[181] but later investigations indicate that sedaDNA can also be extracted from normal marine sediments, dominated by clastic or biogenic mineral fractions.[182][183][184] In addition, the low temperature of deep-sea water (0–4 °C) ensures a good preservation of sedaDNA.[178][180] Using planktonic foraminifera as a "Rosetta Stone", allowing benchmarking of sedaDNA signatures by co-occurring fossil tests of these organisms, Morard et al. showed in 2017 that the fingerprint of plankton eDNA arriving on the seafloor preserves the ecological signature of these organisms at a large geographic scale.[181] This indicates that planktonic community eDNA is deposited onto the seafloor below, together with aggregates, skeletons and other sinking planktonic material. If this is true, sedaDNA should be able to record signatures of surface ocean hydrography, affecting the composition of plankton communities, with the same spatial resolution as the skeletal remains of the plankton. In addition, if the plankton eDNA is arriving on the seafloor in association with aggregates or shells, it is possible that it withstands the transport through the water column by fixation onto mineral surfaces. The same mechanism has been proposed to explain the preservation of sedaDNA in sediments,[182][183][184] implying that the flux of planktonic eDNA encapsulated in calcite test arriving on the seafloor is conditioned for preservation upon burial.[179]
Planktonic foraminifera sedaDNA is an ideal proxy both “horizontally” to assess the spatial resolution of reconstructing past surface ocean hydrographic features and “vertically”, to unambiguously track the burial of its signal throughout the sediment column. Indeed, the flux of planktonic foraminifera eDNA should be proportionate to the flux of dead foraminiferal shells sinking to the seafloor, allowing independent benchmarking of the eDNA signal. eDNA is powerful tool to study ecosystem because it does not require direct taxonomic knowledge thus allowing information to be gathered on every organism present in a sample, even at the cryptic level. However, assignment of the eDNA sequences to known organisms is done via comparison with reference sequences (or barcodes) made available in public repositories or curated databases.[185] The taxonomy of planktonic foraminifera is well understood[186] and barcodes exist allowing almost complete mapping of eDNA amplicons on the taxonomy based on foraminiferal test morphology.[187][188] Importantly, the composition of planktonic foraminifera communities is closely linked to surface hydrography and this signal is preserved by fossil tests deposited on the seafloor.[189][190] Since foraminiferal eDNA accumulated in the ocean sediment can be recovered, it could be used to analyze changes in planktonic and benthic communities over time.[191][192][193][194][179]
On 7 December 2022, The New York Times reported that two-million year old eDNA genetic material was found in Greenland, and is currently considered the oldest DNA discovered so far.[11][12]
Participatory research and citizen science
The relative simplicity of eDNA sampling lends itself to projects which seek to involve local communities in being part of research projects, including collecting and analysing DNA samples. This can empower local communities (including Indigenous peoples) to be actively involved in monitoring the species in an environment, and help make informed decisions as part of participatory action research model. An example of such a project has been demonstrated by the charity Science for All with the 'Wild DNA' project.[195]
See also
- Circulating free DNA
- Exogenous DNA
- Extracellular RNA
- RNAs present in environmental samples
- Shadow Effect (Genetics)
References
- ↑ 1.0 1.1 1.2 1.3 Thomsen, Philip Francis; Sigsgaard, Eva E. (2019). "Environmental DNA metabarcoding of wild flowers reveals diverse communities of terrestrial arthropods". Ecology and Evolution 9 (4): 1665–1679. doi:10.1002/ece3.4809. PMID 30847063. Bibcode: 2019EcoEv...9.1665T.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 2.0 2.1 2.2 Stewart, Kathryn A. (2019-04-01). "Understanding the effects of biotic and abiotic factors on sources of aquatic environmental DNA" (in en). Biodiversity and Conservation 28 (5): 983–1001. doi:10.1007/s10531-019-01709-8. ISSN 1572-9710. Bibcode: 2019BiCon..28..983S. https://doi.org/10.1007/s10531-019-01709-8.
- ↑ "Environmental DNA – how a tool used to detect endangered wildlife ended up helping fight the COVID-19 pandemic". 21 April 2021. https://theconversation.com/environmental-dna-how-a-tool-used-to-detect-endangered-wildlife-ended-up-helping-fight-the-covid-19-pandemic-158286.
- ↑ "What is eDNA?". http://freshwaterhabitats.org.uk/projects/edna/edna/.
- ↑ Fahner, Nicole (2016). "Large-Scale Monitoring of Plants through Environmental DNA Metabarcoding of Soil: Recovery, Resolution, and Annotation of Four DNA Markers". PLOS ONE 11 (6): 1–16. doi:10.1371/journal.pone.0157505. ISSN 1932-6203. PMID 27310720. Bibcode: 2016PLoSO..1157505F.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 Ruppert, Krista M.; Kline, Richard J.; Rahman, Md Saydur (2019). "Past, present, and future perspectives of environmental DNA (EDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA". Global Ecology and Conservation 17: e00547. doi:10.1016/j.gecco.2019.e00547.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Bohmann, Kristine; Evans, Alice; Gilbert, M. Thomas P.; Carvalho, Gary R.; Creer, Simon; Knapp, Michael; Yu, Douglas W.; de Bruyn, Mark (2014-06-01). "Environmental DNA for wildlife biology and biodiversity monitoring". Trends in Ecology & Evolution 29 (6): 358–367. doi:10.1016/j.tree.2014.04.003. ISSN 1872-8383. PMID 24821515.
- ↑ Qu, Chanjuan; Stewart, Kathryn A. (2019-02-18). "Evaluating monitoring options for conservation: comparing traditional and environmental DNA tools for a critically endangered mammal" (in en). The Science of Nature 106 (3): 9. doi:10.1007/s00114-019-1605-1. ISSN 1432-1904. PMID 30778682. Bibcode: 2019SciNa.106....9Q. https://doi.org/10.1007/s00114-019-1605-1.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 Thomsen, Philip Francis; Willerslev, Eske (2015-03-01). "Environmental DNA – An emerging tool in conservation for monitoring past and present biodiversity". Biological Conservation. Special Issue: Environmental DNA: A powerful new tool for biological conservation 183: 4–18. doi:10.1016/j.biocon.2014.11.019.
- ↑ 10.0 10.1 10.2 Tsuji, Satsuki (2016). "Effects of water pH and proteinase K treatment on the yield of environmental DNA from water samples". Limnology 18: 1–7. doi:10.1007/s10201-016-0483-x. ISSN 1439-8621.
- ↑ 11.0 11.1 Zimmer, Carl (7 December 2022). "Oldest Known DNA Offers Glimpse of a Once-Lush Arctic - In Greenland's permafrost, scientists discovered two-million-year-old genetic material from scores of plant and animal species, including mastodons, geese, lemmings and ants.". The New York Times. https://www.nytimes.com/2022/12/07/science/oldest-dna-greenland-species.html. Retrieved 7 December 2022.
- ↑ 12.0 12.1 Kjær, Kurt H. (7 December 2022). "A 2-million-year-old ecosystem in Greenland uncovered by environmental DNA". Nature 612 (7939): 283–291. doi:10.1038/s41586-022-05453-y. PMID 36477129. Bibcode: 2022Natur.612..283K.
- ↑ 13.0 13.1 Ficetola, Gentile Francesco; Miaud, Claude; Pompanon, François; Taberlet, Pierre (2008). "Species detection using environmental DNA from water samples". Biology Letters 4 (4): 423–425. doi:10.1098/rsbl.2008.0118. PMID 18400683.
- ↑ 14.0 14.1 14.2 14.3 Barnes, Matthew A.; Turner, Cameron R. (2016). "The ecology of environmental DNA and implications for conservation genetics". Conservation Genetics 17 (1): 1–17. doi:10.1007/s10592-015-0775-4. Bibcode: 2016ConG...17....1B.
- ↑ Deiner, Kristy; Walser, Jean-Claude; Mächler, Elvira; Altermatt, Florian (2015). "Choice of capture and extraction methods affect detection of freshwater biodiversity from environmental DNA". Biological Conservation 183: 53–63. doi:10.1016/j.biocon.2014.11.018. https://www.dora.lib4ri.ch/eawag/islandora/object/eawag%3A7860.
- ↑ 16.0 16.1 Taberlet, Pierre; Coissac, Eric; Pompanon, François; Brochmann, Christian; Willerslev, Eske (2012). "Towards next-generation biodiversity assessment using DNA metabarcoding". Molecular Ecology 21 (8): 2045–2050. doi:10.1111/j.1365-294X.2012.05470.x. PMID 22486824. Bibcode: 2012MolEc..21.2045T.
- ↑ 17.0 17.1 17.2 17.3 17.4 Stewart, Kathryn; Ma, Hongjuan; Zheng, Jinsong; Zhao, Jianfu (2017). "Using environmental DNA to assess population-wide spatiotemporal reserve use" (in es). Conservation Biology 31 (5): 1173–1182. doi:10.1111/cobi.12910. ISSN 1523-1739. PMID 28221696. Bibcode: 2017ConBi..31.1173S. https://conbio.onlinelibrary.wiley.com/doi/abs/10.1111/cobi.12910.
- ↑ 18.0 18.1 18.2 18.3 Goldberg, Caren S.; Turner, Cameron R.; Deiner, Kristy; Klymus, Katy E.; Thomsen, Philip Francis; Murphy, Melanie A.; Spear, Stephen F.; McKee, Anna et al. (2016). "Critical considerations for the application of environmental DNA methods to detect aquatic species". Methods in Ecology and Evolution 7 (11): 1299–1307. doi:10.1111/2041-210X.12595. Bibcode: 2016MEcEv...7.1299G.
- ↑ 19.0 19.1 19.2 19.3 Hering, Daniel; Borja, Angel; Jones, J.Iwan; Pont, Didier; Boets, Pieter; Bouchez, Agnes; Bruce, Kat; Drakare, Stina et al. (2018). "Implementation options for DNA-based identification into ecological status assessment under the European Water Framework Directive". Water Research 138: 192–205. doi:10.1016/j.watres.2018.03.003. PMID 29602086. Bibcode: 2018WatRe.138..192H. http://qmro.qmul.ac.uk/xmlui/handle/123456789/36293.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 Deiner, Kristy; Bik, Holly M.; Mächler, Elvira; Seymour, Mathew; Lacoursière‐Roussel, Anaïs; Altermatt, Florian; Creer, Simon; Bista, Iliana et al. (2017). "Environmental DNA metabarcoding: Transforming how we survey animal and plant communities". Molecular Ecology 26 (21): 5872–5895. doi:10.1111/mec.14350. PMID 28921802. Bibcode: 2017MolEc..26.5872D.
- ↑ 21.0 21.1 Ma, Hongjuan; Stewart, Kathryn; Lougheed, Stephen; Zheng, Jinsong; Wang, Yuxiang; Zhao, Jianfu (2016-12-01). "Characterization, optimization, and validation of environmental DNA (eDNA) markers to detect an endangered aquatic mammal" (in en). Conservation Genetics Resources 8 (4): 561–568. doi:10.1007/s12686-016-0597-9. ISSN 1877-7260. Bibcode: 2016ConGR...8..561M. https://doi.org/10.1007/s12686-016-0597-9.
- ↑ 22.0 22.1 22.2 Coissac, Eric; Riaz, Tiayyba; Puillandre, Nicolas (2012). "Bioinformatic challenges for DNA metabarcoding of plants and animals". Molecular Ecology 21 (8): 1834–1847. doi:10.1111/j.1365-294X.2012.05550.x. PMID 22486822. Bibcode: 2012MolEc..21.1834C.
- ↑ Eichmiller, Jessica J.; Best, Sendréa E.; Sorensen, Peter W. (2016). "Effects of Temperature and Trophic State on Degradation of Environmental DNA in Lake Water". Environmental Science & Technology 50 (4): 1859–1867. doi:10.1021/acs.est.5b05672. PMID 26771292. Bibcode: 2016EnST...50.1859E.
- ↑ Carew, Melissa E.; Pettigrove, Vincent J.; Metzeling, Leon; Hoffmann, Ary A. (2013). "Environmental monitoring using next generation sequencing: Rapid identification of macroinvertebrate bioindicator species". Frontiers in Zoology 10 (1): 45. doi:10.1186/1742-9994-10-45. PMID 23919569.
- ↑ 25.0 25.1 25.2 25.3 Creer, Simon; Deiner, Kristy; Frey, Serita; Porazinska, Dorota; Taberlet, Pierre; Thomas, W. Kelley; Potter, Caitlin; Bik, Holly M. (2016). "The ecologist's field guide to sequence‐based identification of biodiversity". Methods in Ecology and Evolution 7 (9): 1008–1018. doi:10.1111/2041-210X.12574. Bibcode: 2016MEcEv...7.1008C.
- ↑ "Extracellular self-DNA warns plants of danger". https://www.newphytologist.org/news/view/97.
- ↑ Mazzoleni S, Bonanomi G, Incerti G, Chiusano ML, Termolino P, Mingo A, Senatore M, Giannino F, Cartenì F, Rietkerk M, Lanzotti V. 2015a. Inhibitory and toxic effects of extracellular self-DNA in litter: a mechanism for negative plant–soil feedbacks? New Phytologist 205: 1195–1210
- ↑ Mazzoleni S, Cartenì F, Bonanomi G, Senatore M, Termolino P, Giannino F, Incerti G, Rietkerk M, Lanzotti V, Chiusano ML. 2015b. Inhibitory effects of extracellular self-DNA: a general biological process? New Phytologist 206: 127–132.
- ↑ Veresoglou, SD, Aguilar-Trigueros, CA, Mansour, I, Rillig, MC 2015. Self-DNA: a blessing in disguise? New Phytologist 207: 488–490.
- ↑ Duran-Flores, D, Heil, M. 2015. Growth inhibition by self-DNA: a phenomenon and its multiple explanations. New Phytologist 207: 482–485.
- ↑ Patent WO 2014/020624 A9
- ↑ 32.0 32.1 32.2 Qu, Chanjuan; Stewart, Kathryn A.; Clemente-Carvalho, Rute; Zheng, Jinsong; Wang, Yuxiang; Gong, Cheng; Ma, Limin; Zhao, Jianfu et al. (2020-10-07). "Comparing fish prey diversity for a critically endangered aquatic mammal in a reserve and the wild using eDNA metabarcoding" (in en). Scientific Reports 10 (1): 16715. doi:10.1038/s41598-020-73648-2. ISSN 2045-2322. PMID 33028872.
- ↑ Banerjee, Pritam; Stewart, Kathryn A.; Antognazza, Caterina M.; Bunholi, Ingrid V.; Deiner, Kristy; Barnes, Matthew A.; Saha, Santanu; Verdier, Héloïse et al. (2022). "Plant–animal interactions in the era of environmental DNA (eDNA)—A review". Environmental DNA 4 (5): 987–999. doi:10.1002/edn3.308. https://onlinelibrary.wiley.com/doi/full/10.1002/edn3.308.
- ↑ Ficetola, Gentile Francesco; Taberlet, Pierre; Coissac, Eric (2016). "How to limit false positives in environmental DNA and metabarcoding?". Molecular Ecology Resources 16 (3): 604–607. doi:10.1111/1755-0998.12508. PMID 27062589.
- ↑ Yu, Douglas W.; Ji, Yinqiu; Emerson, Brent C.; Wang, Xiaoyang; Ye, Chengxi; Yang, Chunyan; Ding, Zhaoli (2012). "Biodiversity soup: Metabarcoding of arthropods for rapid biodiversity assessment and biomonitoring". Methods in Ecology and Evolution 3 (4): 613–623. doi:10.1111/j.2041-210X.2012.00198.x. Bibcode: 2012MEcEv...3..613Y.
- ↑ Cristescu, Melania E. (2014). "From barcoding single individuals to metabarcoding biological communities: Towards an integrative approach to the study of global biodiversity". Trends in Ecology & Evolution 29 (10): 566–571. doi:10.1016/j.tree.2014.08.001. PMID 25175416.
- ↑ Gibson, Joel F.; Shokralla, Shadi; Curry, Colin; Baird, Donald J.; Monk, Wendy A.; King, Ian; Hajibabaei, Mehrdad (2015). "Large-Scale Biomonitoring of Remote and Threatened Ecosystems via High-Throughput Sequencing". PLOS ONE 10 (10): e0138432. doi:10.1371/journal.pone.0138432. PMID 26488407. Bibcode: 2015PLoSO..1038432G.
- ↑ Chua, Physilia Y. S.; Bourlat, Sarah J.; Ferguson, Cameron; Korlevic, Petra; Zhao, Leia; Ekrem, Torbjørn; Meier, Rudolf; Lawniczak, Mara K. N. (10 March 2023). "Future of DNA-based insect monitoring". Trends in Genetics 39 (7): 531–544. doi:10.1016/j.tig.2023.02.012. PMID 36907721.
- ↑ 39.0 39.1 Lennon, J. T.; Muscarella, M. E.; Placella, S. A.; Lehmkuhl, B. K. (2018). "How, when, and Where Relic DNA Affects Microbial Diversity". mBio 9 (3). doi:10.1128/mBio.00637-18. PMID 29921664.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Tani, Katsuji; Nasu, Masao (2010). "Roles of Extracellular DNA in Bacterial Ecosystems". Extracellular Nucleic Acids. Springer. pp. 25–38. ISBN 978-3-642-12616-1. https://archive.org/details/extracellularnuc00kiku.
- ↑ "Extracellular nucleic acids". BioEssays 29 (7): 654–67. July 2007. doi:10.1002/bies.20604. PMID 17563084.
- ↑ "DNA as a nutrient: novel role for bacterial competence gene homologs". Journal of Bacteriology 183 (21): 6288–93. November 2001. doi:10.1128/JB.183.21.6288-6293.2001. PMID 11591672.
- ↑ "Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms". PLOS Pathogens 4 (11): e1000213. November 2008. doi:10.1371/journal.ppat.1000213. PMID 19023416.
- ↑ "A bacterial extracellular DNA inhibits settling of motile progeny cells within a biofilm". Molecular Microbiology 77 (4): 815–29. August 2010. doi:10.1111/j.1365-2958.2010.07267.x. PMID 20598083.
- ↑ "Extracellular DNA required for bacterial biofilm formation". Science 295 (5559): 1487. February 2002. doi:10.1126/science.295.5559.1487. PMID 11859186.
- ↑ "DNA builds and strengthens the extracellular matrix in Myxococcus xanthus biofilms by interacting with exopolysaccharides". PLOS ONE 7 (12): e51905. 2012. doi:10.1371/journal.pone.0051905. PMID 23300576. Bibcode: 2012PLoSO...751905H.
- ↑ "Investigating the potential use of environmental DNA (eDNA) for genetic monitoring of marine mammals". PLOS ONE 7 (8): e41781. 2012. doi:10.1371/journal.pone.0041781. PMID 22952587. Bibcode: 2012PLoSO...741781F.
- ↑ "Researchers Detect Land Animals Using DNA in Nearby Water Bodies". https://www.the-scientist.com/news-opinion/researchers-detect-land-animals-using-dna-in-nearby-water-bodies-67481?fbclid=IwAR0OQQEgFNQUG_hXie6P2uUPhEchv9FPDJSUqrjah3eur7275uaCSB0GzYE#.Xqk9eRnC76M.facebook.
- ↑ Nunn, Jack S.; Shafee, Thomas (2022). "Standardised Data on Initiatives – STARDIT: Beta Version". OSF Preprints 8 (1): 31. doi:10.31219/osf.io/w5xj6. PMID 35854364. PMC 9294764. https://doi.org/10.31219/osf.io/w5xj6.
- ↑ 50.0 50.1 Armbrecht, Linda (2020). "The Potential of Sedimentary Ancient DNA to Reconstruct Past Ocean Ecosystems". Oceanography 33 (2). doi:10.5670/oceanog.2020.211.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 51.0 51.1 Bass, David (2015). "Diverse Applications of Environmental DNA Methods in Parasitology". Trends in Parasitology 31 (10): 499–513. doi:10.1016/j.pt.2015.06.013. PMID 26433253.
- ↑ Willerslev, Eske; Hansen, Anders J.; Binladen, Jonas; Brand, Tina B.; Gilbert, M. Thomas fP.; Shapiro, Beth; Bunce, Michael; Wiuf, Carsten et al. (2003-05-02). "Diverse Plant and Animal Genetic Records from Holocene and Pleistocene Sediments". Science 300 (5620): 791–795. doi:10.1126/science.1084114. ISSN 0036-8075. PMID 12702808. Bibcode: 2003Sci...300..791W.
- ↑ Andersen, Kenneth; Bird, Karen Lise; Rasmussen, Morten; Haile, James; Breuning-Madsen, Henrik; Kjaer, Kurt H.; Orlando, Ludovic; Gilbert, M. Thomas P. et al. (2012-04-01). "Meta-barcoding of 'dirt' DNA from soil reflects vertebrate biodiversity". Molecular Ecology 21 (8): 1966–1979. doi:10.1111/j.1365-294X.2011.05261.x. ISSN 1365-294X. PMID 21917035. Bibcode: 2012MolEc..21.1966A.
- ↑ Nunn, Jack (2020). "Science for All - Publicly funded research report (June 2018-December 2019)" (in en-US). Figshare. doi:10.26181/5eba630a64e08. https://doi.org/10.26181/5eba630a64e08.
- ↑ Turner, Cameron R. (2014). "Fish environmental DNA is more concentrated in aquatic sediments than surface water". Biological Conservation 183: 93–102. doi:10.1016/j.biocon.2014.11.017. ISSN 0006-3207.
- ↑ Gong; Fan; Li; Li; Zhang; Gromig; Smith; Dummann et al. (2019). "Coring of Antarctic Subglacial Sediments". Journal of Marine Science and Engineering 7 (6): 194. doi:10.3390/jmse7060194.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 57.0 57.1 Schultz, Martin (2015). "Modeling the Sensitivity of Field Surveys for Detection of Environmental DNA (eDNA)". PLOS ONE 10 (10): 1–16. doi:10.1371/journal.pone.0141503. ISSN 1932-6203. PMID 26509674. Bibcode: 2015PLoSO..1041503S.
- ↑ 58.0 58.1 Littlefair, Joanne E.; Hrenchuk, Lee E.; Blanchfield, Paul J.; Rennie, Michael D.; Cristescu, Melania E. (2020-04-26). "Thermal stratification and fish thermal preference explain vertical eDNA distributions in lakes" (in en). bioRxiv 30 (13): 3083–3096. doi:10.1101/2020.04.21.042820. PMID 32888228. https://www.biorxiv.org/content/10.1101/2020.04.21.042820v1.
- ↑ "How and Why Lakes Stratify and Turn Over: We explain the science". 2018-05-16. https://www.iisd.org/ela/blog/commentary/lakes-stratify-turn-explain-science-behind-phenomena/.
- ↑ 60.0 60.1 Stoeckle, Bernhard (2016). "Environmental DNA as a monitoring tool for the endangered freshwater pearl mussel (Margaritifera margaritifera L.): a substitute for classical monitoring approaches?". Aquatic Conservation: Marine and Freshwater Ecosystems 26 (6): 1120–1129. doi:10.1002/aqc.2611. Bibcode: 2016ACMFE..26.1120S.
- ↑ 61.0 61.1 Souza, Lesley (2016). "Environmental DNA (eDNA) Detection Probability Is Influenced by Seasonal Activity of Organisms". PLOS ONE 11 (10): 1–15. doi:10.1371/journal.pone.0165273. ISSN 1932-6203. PMID 27776150. Bibcode: 2016PLoSO..1165273D.
- ↑ Saeko, Matsuhashi (2016). "Evaluation of the Environmental DNA Method for Estimating Distribution and Biomass of Submerged Aquatic Plants". PLOS ONE 11 (6): 1–14. doi:10.1371/journal.pone.0156217. ISSN 1932-6203. PMID 27304876. Bibcode: 2016PLoSO..1156217M.
- ↑ Tedersoo, Leho; Bahram, Mohammad; Põlme, Sergei; Kõljalg, Urmas; Yorou, Nourou S.; Wijesundera, Ravi; Ruiz, Luis Villarreal; Vasco-Palacios, Aída M. et al. (2014-11-28). "Global diversity and geography of soil fungi". Science 346 (6213): 1256688. doi:10.1126/science.1256688. ISSN 0036-8075. PMID 25430773. https://e-space.mmu.ac.uk/606982/1/LehoFungiFinal.pdf.
- ↑ Detheridge, Andrew Paul; Comont, David; Callaghan, Tony Martin; Bussell, Jennifer; Brand, Graham; Gwynn-Jones, Dylan; Scullion, John; Griffith, Gareth Wyn (June 2018). "Vegetation and edaphic factors influence rapid establishment of distinct fungal communities on former coal-spoil sites". Fungal Ecology 33: 92–103. doi:10.1016/j.funeco.2018.02.002. ISSN 1754-5048.
- ↑ Jones, Rhys Aled; Brophy, Peter M.; Davis, Chelsea N.; Davies, Teri E.; Emberson, Holly; Rees Stevens, Pauline; Williams, Hefin Wyn (2018-06-08). "Detection of Galba truncatula, Fasciola hepatica and Calicophoron daubneyi environmental DNA within water sources on pasture land, a future tool for fluke control?". Parasites & Vectors 11 (1): 342. doi:10.1186/s13071-018-2928-z. ISSN 1756-3305. PMID 29884202.
- ↑ 66.0 66.1 66.2 Bergman, Paul S.; Schumer, Gregg; Blankenship, Scott; Campbell, Elizabeth (2016). "Detection of Adult Green Sturgeon Using Environmental DNA Analysis". PLOS ONE 11 (4): 1–8. doi:10.1371/journal.pone.0153500. ISSN 1932-6203. PMID 27096433. Bibcode: 2016PLoSO..1153500B.
- ↑ "A Guide to Environmental DNA (eDNA) by Biomeme". https://biomeme.com/environmental-dna/.
- ↑ Wang, Xinkun (2016). Next-generation Sequencing Data Analysis. Boca Raton: CRC Press. ISBN 9781482217889. OCLC 940961529.
- ↑ Seymour, Mathew; Durance, Isabelle; Cosby, Bernard J.; Ransom-Jones, Emma; Deiner, Kristy; Ormerod, Steve J.; Colbourne, John K.; Wilgar, Gregory et al. (2018-01-22). "Acidity promotes degradation of multi-species environmental DNA in lotic mesocosms". Communications Biology 1 (1): 4. doi:10.1038/s42003-017-0005-3. ISSN 2399-3642. PMID 30271891.
- ↑ Collins, Rupert A.; Wangensteen, Owen S.; O’Gorman, Eoin J.; Mariani, Stefano; Sims, David W.; Genner, Martin J. (2018-11-05). "Persistence of environmental DNA in marine systems". Communications Biology 1 (1): 185. doi:10.1038/s42003-018-0192-6. ISSN 2399-3642. PMID 30417122.
- ↑ "The TripleLock™ Platform - Precision Biomonitoriong" (in en-CA). https://precisionbiomonitoring.com/triplelock-platform/.
- ↑ Hunter, Margaret E.; Dorazio, Robert M.; Butterfield, John S. S.; Meigs-Friend, Gaia; Nico, Leo G.; Ferrante, Jason A. (2016-11-20). "Detection limits of quantitative and digital PCR assays and their influence in presence-absence surveys of environmental DNA". Molecular Ecology Resources 17 (2): 221–229. doi:10.1111/1755-0998.12619. ISSN 1755-098X. PMID 27768244.
- ↑ Williams, Molly-Ann; O'Grady, Joyce; Ball, Bernard; Carlsson, Jens; Eyto, Elvira de; McGinnity, Philip; Jennings, Eleanor; Regan, Fiona et al. (2019). "The application of CRISPR-Cas for single species identification from environmental DNA" (in en). Molecular Ecology Resources 19 (5): 1106–1114. doi:10.1111/1755-0998.13045. ISSN 1755-0998. PMID 31177615. http://doras.dcu.ie/24151/.
- ↑ Opportunities in Ocean Sciences. Washington, D.C.: National Academies Press. 1998-01-01. doi:10.17226/9500. ISBN 9780309582926.
- ↑ Collen, Ben; (Zoologist), Monika Böhm; Kemp, Rachael; Baillie, Jonathan (2012). Spineless: Status and Trends of the World's Invertebrates. ISBN 9780900881701. https://books.google.com/books?id=xYyOnQAACAAJ&q=%22Spineless:+status+and+trends+of+the+world%27s+invertebrates%22.
- ↑ Dirzo, R.; Young, H. S.; Galetti, M.; Ceballos, G.; Isaac, N. J. B.; Collen, B. (2014). "Defaunation in the Anthropocene". Science 345 (6195): 401–406. doi:10.1126/science.1251817. PMID 25061202. Bibcode: 2014Sci...345..401D. http://discovery.ucl.ac.uk/1436030/1/Collen_Dirzo%20etal%202014%20Science%20Accepted.pdf.
- ↑ European Red List of Bees. Publications Office. 2014. ISBN 9789279445125. https://books.google.com/books?id=87OPjwEACAAJ&q=%22European+red+list+of+bees%22.
- ↑ Swaay, Chris van (2010). European Red List of Butterflies. IUCN (International Union for Conservation of Nature). ISBN 9789279141515. https://books.google.com/books?id=Sz81cgAACAAJ&q=%22European+red+list+of+butterflies%22.
- ↑ Stork, Nigel E. (2018). "How Many Species of Insects and Other Terrestrial Arthropods Are There on Earth?". Annual Review of Entomology 63: 31–45. doi:10.1146/annurev-ento-020117-043348. PMID 28938083.
- ↑ Habel, Jan Christian; Dengler, Jürgen; Janišová, Monika; Török, Péter; Wellstein, Camilla; Wiezik, Michal (2013). "European grassland ecosystems: Threatened hotspots of biodiversity". Biodiversity and Conservation 22 (10): 2131–2138. doi:10.1007/s10531-013-0537-x. Bibcode: 2013BiCon..22.2131H.
- ↑ Joern, Anthony; Laws, Angela N. (2013). "Ecological Mechanisms Underlying Arthropod Species Diversity in Grasslands". Annual Review of Entomology 58: 19–36. doi:10.1146/annurev-ento-120811-153540. PMID 22830354.
- ↑ Silva, João Pedro (2008). LIFE and Europe's Grasslands: Restoring a Forgotten Habitat. Office for Official Publications of the Europ. Communities. ISBN 9789279101595. https://books.google.com/books?id=t8n3SAAACAAJ&q=%22LIFE+and+Europe%E2%80%99s+grasslands.+Restoring+a+forgotten+habitat.%22.
- ↑ Biesmeijer, J. C.; Roberts, S. P.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A. P.; Potts, S. G. et al. (2006). "Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands". Science 313 (5785): 351–354. doi:10.1126/science.1127863. PMID 16857940. Bibcode: 2006Sci...313..351B.
- ↑ Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E. L. (2015). "Bee declines driven by combined stress from parasites, pesticides, and lack of flowers". Science 347 (6229). doi:10.1126/science.1255957. PMID 25721506.
- ↑ Potts, Simon G.; Biesmeijer, Jacobus C.; Kremen, Claire; Neumann, Peter; Schweiger, Oliver; Kunin, William E. (2010). "Global pollinator declines: Trends, impacts and drivers". Trends in Ecology & Evolution 25 (6): 345–353. doi:10.1016/j.tree.2010.01.007. PMID 20188434.
- ↑ European Environment Agency (2013). The European grassland butterfly indicator: 1990–2011. Publications Office. doi:10.2800/89760. ISBN 9789292134020.
- ↑ Ollerton, Jeff; Winfree, Rachael; Tarrant, Sam (2011). "How many flowering plants are pollinated by animals?". Oikos 120 (3): 321–326. doi:10.1111/j.1600-0706.2010.18644.x. Bibcode: 2011Oikos.120..321O.
- ↑ Price, Peter W.; Denno, Robert F.; Eubanks, Micky D.; Finke, Deborah L.; Kaplan, Ian (2011). Insect Ecology. doi:10.1017/CBO9780511975387. ISBN 9780511975387.
- ↑ Hopkins, G. W.; Freckleton, R. P. (2002). "Declines in the numbers of amateur and professional taxonomists: Implications for conservation". Animal Conservation 5 (3): 245–249. doi:10.1017/S1367943002002299. Bibcode: 2002AnCon...5..245H.
- ↑ Sangster, George; Luksenburg, Jolanda A. (2015). "Declining Rates of Species Described per Taxonomist: Slowdown of Progress or a Side-effect of Improved Quality in Taxonomy?". Systematic Biology 64 (1): 144–151. doi:10.1093/sysbio/syu069. PMID 25190593.
- ↑ Wheeler, Q. D.; Raven, P. H.; Wilson, E. O. (2004). "Taxonomy: Impediment or Expedient?". Science 303 (5656): 285. doi:10.1126/science.303.5656.285. PMID 14726557.
- ↑ Bush, Alex; Sollmann, Rahel; Wilting, Andreas; Bohmann, Kristine; Cole, Beth; Balzter, Heiko; Martius, Christopher; Zlinszky, András et al. (2017). "Connecting Earth observation to high-throughput biodiversity data". Nature Ecology & Evolution 1 (7): 176. doi:10.1038/s41559-017-0176. PMID 28812589. Bibcode: 2017NatEE...1..176B. http://nora.nerc.ac.uk/id/eprint/519336/1/N519336PP.pdf.
- ↑ Arribas, Paula; Andújar, Carmelo; Hopkins, Kevin; Shepherd, Matthew; Vogler, Alfried P. (2016). "Metabarcoding and mitochondrial metagenomics of endogean arthropods to unveil the mesofauna of the soil". Methods in Ecology and Evolution 7 (9): 1071–1081. doi:10.1111/2041-210X.12557. Bibcode: 2016MEcEv...7.1071A.
- ↑ Elbrecht, Vasco; Taberlet, Pierre; Dejean, Tony; Valentini, Alice; Usseglio-Polatera, Philippe; Beisel, Jean-Nicolas; Coissac, Eric; Boyer, Frederic et al. (2016). "Testing the potential of a ribosomal 16S marker for DNA metabarcoding of insects". PeerJ 4: e1966. doi:10.7717/peerj.1966. PMID 27114891.
- ↑ Hajibabaei, Mehrdad; Shokralla, Shadi; Zhou, Xin; Singer, Gregory A. C.; Baird, Donald J. (2011). "Environmental Barcoding: A Next-Generation Sequencing Approach for Biomonitoring Applications Using River Benthos". PLOS ONE 6 (4): e17497. doi:10.1371/journal.pone.0017497. PMID 21533287. Bibcode: 2011PLoSO...617497H.
- ↑ Xu, Charles C. Y.; Yen, Ivy J.; Bowman, Dean; Turner, Cameron R. (2015). "Spider Web DNA: A New Spin on Noninvasive Genetics of Predator and Prey". PLOS ONE 10 (11): e0142503. doi:10.1371/journal.pone.0142503. PMID 26606730. Bibcode: 2015PLoSO..1042503X.
- ↑ Derocles, Stéphane A. P.; Evans, Darren M.; Nichols, Paul C.; Evans, S. Aifionn; Lunt, David H. (2015). "Determining Plant – Leaf Miner – Parasitoid Interactions: A DNA Barcoding Approach". PLOS ONE 10 (2): e0117872. doi:10.1371/journal.pone.0117872. PMID 25710377. Bibcode: 2015PLoSO..1017872D.
- ↑ Blake, Max; McKeown, Niall J.; Bushell, Mark L. T.; Shaw, Paul W. (2016). "DNA extraction from spider webs". Conservation Genetics Resources 8 (3): 219–221. doi:10.1007/s12686-016-0537-8. Bibcode: 2016ConGR...8..219B.
- ↑ Bittleston, Leonora S.; Baker, Christopher C. M.; Strominger, Lila B.; Pringle, Anne; Pierce, Naomi E. (2016). "Metabarcoding as a tool for investigating arthropod diversity in Nepenthespitcher plants". Austral Ecology 41 (2): 120–132. doi:10.1111/aec.12271. Bibcode: 2016AusEc..41..120B.
- ↑ Gamonal Gomez, Nerea; Sørensen, Didde Hedegaard; Chua, Physilia Ying Shi; Sigsgaard, Lene (2022-12-06). "Assessing flower‐visiting arthropod diversity in apple orchards through metabarcoding of environmental <scp>DNA</scp> from flowers and visual census". Environmental DNA. doi:10.1002/edn3.362. ISSN 2637-4943. http://dx.doi.org/10.1002/edn3.362.
- ↑ 101.0 101.1 Taberlet, Pierre; Coissac, Eric; Hajibabaei, Mehrdad; Rieseberg, Loren H. (2012). "Environmental DNA". Molecular Ecology 21 (8): 1789–1793. doi:10.1111/j.1365-294X.2012.05542.x. PMID 22486819. Bibcode: 2012MolEc..21.1789T.
- ↑ Thomsen, Philip Francis; Kielgast, JOS; Iversen, Lars L.; Wiuf, Carsten; Rasmussen, Morten; Gilbert, M. Thomas P.; Orlando, Ludovic; Willerslev, Eske (2012). "Monitoring endangered freshwater biodiversity using environmental DNA". Molecular Ecology 21 (11): 2565–2573. doi:10.1111/j.1365-294X.2011.05418.x. PMID 22151771. Bibcode: 2012MolEc..21.2565T.
- ↑ Zinger, Lucie; Taberlet, Pierre; Schimann, Heidy; Bonin, Aurélie; Boyer, Frédéric; De Barba, Marta; Gaucher, Philippe; Gielly, Ludovic et al. (2019). "Body size determines soil community assembly in a tropical forest". Molecular Ecology 28 (3): 528–543. doi:10.1111/mec.14919. PMID 30375061. Bibcode: 2019MolEc..28..528Z.
- ↑ Jurado-Rivera, José A.; Vogler, Alfried P.; Reid, Chris A.M; Petitpierre, Eduard; Gómez-Zurita, Jesús (2009). "DNA barcoding insect–host plant associations". Proceedings of the Royal Society B: Biological Sciences 276 (1657): 639–648. doi:10.1098/rspb.2008.1264. PMID 19004756.
- ↑ Paula, Débora P.; Linard, Benjamin; Crampton-Platt, Alex; Srivathsan, Amrita; Timmermans, Martijn J. T. N.; Sujii, Edison R.; Pires, Carmen S. S.; Souza, Lucas M. et al. (2016). "Uncovering Trophic Interactions in Arthropod Predators through DNA Shotgun-Sequencing of Gut Contents". PLOS ONE 11 (9): e0161841. doi:10.1371/journal.pone.0161841. PMID 27622637. Bibcode: 2016PLoSO..1161841P.
- ↑ Bohmann, Kristine; Monadjem, Ara; Lehmkuhl Noer, Christina; Rasmussen, Morten; Zeale, Matt R. K.; Clare, Elizabeth; Jones, Gareth; Willerslev, Eske et al. (2011). "Molecular Diet Analysis of Two African Free-Tailed Bats (Molossidae) Using High Throughput Sequencing". PLOS ONE 6 (6): e21441. doi:10.1371/journal.pone.0021441. PMID 21731749. Bibcode: 2011PLoSO...621441B.
- ↑ Vesterinen, Eero J.; Lilley, Thomas; Laine, Veronika N.; Wahlberg, Niklas (2013). "Next Generation Sequencing of Fecal DNA Reveals the Dietary Diversity of the Widespread Insectivorous Predator Daubenton's Bat (Myotis daubentonii) in Southwestern Finland". PLOS ONE 8 (11): e82168. doi:10.1371/journal.pone.0082168. PMID 24312405. Bibcode: 2013PLoSO...882168V.
- ↑ Bell, Karen L.; Fowler, Julie; Burgess, Kevin S.; Dobbs, Emily K.; Gruenewald, David; Lawley, Brice; Morozumi, Connor; Brosi, Berry J. (2017). "Applying Pollen DNA Metabarcoding to the Study of Plant–Pollinator Interactions". Applications in Plant Sciences 5 (6). doi:10.3732/apps.1600124. PMID 28690929.
- ↑ Pornon, André; Escaravage, Nathalie; Burrus, Monique; Holota, Hélène; Khimoun, Aurélie; Mariette, Jérome; Pellizzari, Charlène; Iribar, Amaia et al. (2016). "Using metabarcoding to reveal and quantify plant-pollinator interactions". Scientific Reports 6: 27282. doi:10.1038/srep27282. PMID 27255732. Bibcode: 2016NatSR...627282P.
- ↑ Taberlet, Pierre (2018). Environmental DNA : for biodiversity research and monitoring. Oxford. ISBN 9780198767220.
- ↑ "WWF's Arnaud Lyet on measuring wildlife populations". World Wildlife Fund. https://www.worldwildlife.org/magazine/issues/summer-2016/articles/wwf-s-arnaud-lyet-on-measuring-wildlife-populations.
- ↑ "eDNA – Not just for fisheries biologists anymore". 2017-12-08. http://wildlife.org/not-just-for-fisheries-biologists-anymore/.
- ↑ Roth, Annie (2018-11-19). "How DNA from snow helps scientists track elusive animals". National Geographic. https://www.nationalgeographic.com/animals/2018/11/environmental-dna-snow-helps-track-lynx-rare-animals/.
- ↑ Franklin, Thomas W.; McKelvey, Kevin S.; Golding, Jessie D.; Mason, Daniel H.; Dysthe, Joseph C.; Pilgrim, Kristine L.; Squires, John R.; Aubry, Keith B. et al. (2019). "Using environmental DNA methods to improve winter surveys for rare carnivores: DNA from snow and improved noninvasive techniques". Biological Conservation 229: 50–58. doi:10.1016/j.biocon.2018.11.006.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Lynggaard, Christina; Bertelsen, Mads Frost; Jensen, Casper V.; Johnson, Matthew S.; Frøslev, Tobias Guldberg; Olsen, Morten Tange; Bohmann, Kristine (6 January 2022). "Airborne environmental DNA for terrestrial vertebrate community monitoring". Current Biology 32 (3): 701–707.e5. doi:10.1016/j.cub.2021.12.014. PMID 34995490.
- ↑ Clare, Elizabeth L.; Economou, Chloe K.; Bennett, Frances J.; Dyer, Caitlin E.; Adams, Katherine; McRobie, Benjamin; Drinkwater, Rosie; Littlefair, Joanne E. (January 2022). "Measuring biodiversity from DNA in the air". Current Biology 32 (3): 693–700.e5. doi:10.1016/j.cub.2021.11.064. PMID 34995488.
- ↑ Clare, Elizabeth L.; Economou, Chloe K.; Faulkes, Chris G.; Gilbert, James D.; Bennett, Frances; Drinkwater, Rosie; Littlefair, Joanne E. (2021). "EDNAir: Proof of concept that animal DNA can be collected from air sampling". PeerJ 9: e11030. doi:10.7717/peerj.11030. PMID 33850648.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Researchers can now collect and sequence DNA from the air Live Science , 6 April 2021.
- ↑ Métris, Kimberly L.; Métris, Jérémy (2023-04-14). "Aircraft surveys for air eDNA: probing biodiversity in the sky" (in en). PeerJ 11: e15171. doi:10.7717/peerj.15171. ISSN 2167-8359. PMID 37077310.
- ↑ Kenneth T. Frank; Brian Petrie; Jae S. Choi; William C. Leggett (2005). "Trophic Cascades in a Formerly Cod-Dominated Ecosystem". Science 308 (5728): 1621–1623. doi:10.1126/science.1113075. PMID 15947186. Bibcode: 2005Sci...308.1621F.
- ↑ Walters, Carl; Maguire, Jean-Jacques (1996). "Lessons for stock assessment from the northern cod collapse". Reviews in Fish Biology and Fisheries 6 (2). doi:10.1007/BF00182340.
- ↑ ICES (2018). NEAFC request on updated advice for cod (Gadus morhua) in Subdivision 5.b.1 (Faroe Plateau). doi:10.17895/ices.pub.4651. https://www.ices.dk/sites/pub/Publication%20Reports/Advice/2018/Special_requests/neafc.2018.31a.pdf.
- ↑ Heessen, Henk J. L.; Daan, Niels; Ellis, Jim R. (September 2015). Fish atlas of the Celtic Sea, North Sea, and Baltic Sea: Based on international research-vessel surveys. Brill. ISBN 9789086868780. https://books.google.com/books?id=nBTTDwAAQBAJ&q=%22Fish+Atlas+of+the+Celtic+Sea,+North+Sea+and+Baltic+Sea%22&pg=PA497.
- ↑ Pusceddu, A.; Bianchelli, S.; Martin, J.; Puig, P.; Palanques, A.; Masque, P.; Danovaro, R. (2014). "Chronic and intensive bottom trawling impairs deep-sea biodiversity and ecosystem functioning". Proceedings of the National Academy of Sciences 111 (24): 8861–8866. doi:10.1073/pnas.1405454111. PMID 24843122. Bibcode: 2014PNAS..111.8861P.
- ↑ Arreguin-sanchez, Francisco (1996). "Catchability: A key parameter for fish stock assessment". Reviews in Fish Biology and Fisheries 6 (2). doi:10.1007/BF00182344.
- ↑ 126.0 126.1 126.2 126.3 Salter, Ian; Joensen, Mourits; Kristiansen, Regin; Steingrund, Petur; Vestergaard, Poul (2019). "Environmental DNA concentrations are correlated with regional biomass of Atlantic cod in oceanic waters". Communications Biology 2: 461. doi:10.1038/s42003-019-0696-8. PMID 31840106.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Goldberg, Caren S.; Pilliod, David S.; Arkle, Robert S.; Waits, Lisette P. (2011). "Molecular Detection of Vertebrates in Stream Water: A Demonstration Using Rocky Mountain Tailed Frogs and Idaho Giant Salamanders". PLOS ONE 6 (7): e22746. doi:10.1371/journal.pone.0022746. PMID 21818382. Bibcode: 2011PLoSO...622746G.
- ↑ Valentini, Alice; Taberlet, Pierre; Miaud, Claude; Civade, Raphaël; Herder, Jelger; Thomsen, Philip Francis; Bellemain, Eva; Besnard, Aurélien et al. (2016). "Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding". Molecular Ecology 25 (4): 929–942. doi:10.1111/mec.13428. PMID 26479867. Bibcode: 2016MolEc..25..929V. http://oatao.univ-toulouse.fr/23485/1/Valentini_23485.pdf.
- ↑ Goodwin, Kelly D.; Thompson, Luke R.; Duarte, Bernardo; Kahlke, Tim; Thompson, Andrew R.; Marques, João C.; Caçador, Isabel (2017). "DNA Sequencing as a Tool to Monitor Marine Ecological Status". Frontiers in Marine Science 4. doi:10.3389/fmars.2017.00107.
- ↑ Stoeckle, Mark Y.; Soboleva, Lyubov; Charlop-Powers, Zachary (2017). "Aquatic environmental DNA detects seasonal fish abundance and habitat preference in an urban estuary". PLOS ONE 12 (4): e0175186. doi:10.1371/journal.pone.0175186. PMID 28403183. Bibcode: 2017PLoSO..1275186S.
- ↑ Thomsen, Philip Francis; Kielgast, Jos; Iversen, Lars Lønsmann; Møller, Peter Rask; Rasmussen, Morten; Willerslev, Eske (2012). "Detection of a Diverse Marine Fish Fauna Using Environmental DNA from Seawater Samples". PLOS ONE 7 (8): e41732. doi:10.1371/journal.pone.0041732. PMID 22952584. Bibcode: 2012PLoSO...741732T.
- ↑ 132.0 132.1 Thomsen, Philip Francis; Møller, Peter Rask; Sigsgaard, Eva Egelyng; Knudsen, Steen Wilhelm; Jørgensen, Ole Ankjær; Willerslev, Eske (2016). "Environmental DNA from Seawater Samples Correlate with Trawl Catches of Subarctic, Deepwater Fishes". PLOS ONE 11 (11): e0165252. doi:10.1371/journal.pone.0165252. PMID 27851757. Bibcode: 2016PLoSO..1165252T.
- ↑ 133.0 133.1 Sigsgaard, Eva Egelyng; Nielsen, Ida Broman; Carl, Henrik; Krag, Marcus Anders; Knudsen, Steen Wilhelm; Xing, Yingchun; Holm-Hansen, Tore Hejl; Møller, Peter Rask et al. (2017). "Seawater environmental DNA reflects seasonality of a coastal fish community". Marine Biology 164 (6): 128. doi:10.1007/s00227-017-3147-4. Bibcode: 2017MarBi.164..128S.
- ↑ Takahara, Teruhiko; Minamoto, Toshifumi; Yamanaka, Hiroki; Doi, Hideyuki; Kawabata, Zen'Ichiro (2012). "Estimation of Fish Biomass Using Environmental DNA". PLOS ONE 7 (4): e35868. doi:10.1371/journal.pone.0035868. PMID 22563411. Bibcode: 2012PLoSO...735868T.
- ↑ Doi, Hideyuki; Uchii, Kimiko; Takahara, Teruhiko; Matsuhashi, Saeko; Yamanaka, Hiroki; Minamoto, Toshifumi (2015). "Use of Droplet Digital PCR for Estimation of Fish Abundance and Biomass in Environmental DNA Surveys". PLOS ONE 10 (3): e0122763. doi:10.1371/journal.pone.0122763. PMID 25799582. Bibcode: 2015PLoSO..1022763D.
- ↑ Lacoursière-Roussel, Anaïs; Rosabal, Maikel; Bernatchez, Louis (2016). "Estimating fish abundance and biomass from eDNA concentrations: Variability among capture methods and environmental conditions". Molecular Ecology Resources 16 (6): 1401–1414. doi:10.1111/1755-0998.12522. PMID 26946353.
- ↑ Maruyama, Atsushi; Nakamura, Keisuke; Yamanaka, Hiroki; Kondoh, Michio; Minamoto, Toshifumi (2014). "The Release Rate of Environmental DNA from Juvenile and Adult Fish". PLOS ONE 9 (12): e114639. doi:10.1371/journal.pone.0114639. PMID 25479160. Bibcode: 2014PLoSO...9k4639M.
- ↑ Klymus, Katy E.; Richter, Catherine A.; Chapman, Duane C.; Paukert, Craig (2015). "Quantification of eDNA shedding rates from invasive bighead carp Hypophthalmichthys nobilis and silver carp Hypophthalmichthys molitrix". Biological Conservation 183: 77–84. doi:10.1016/j.biocon.2014.11.020.
- ↑ Li, Jianlong; Lawson Handley, Lori J.; Harper, Lynsey R.; Brys, Rein; Watson, Hayley V.; Di Muri, Cristina; Zhang, Xiang; Hänfling, Bernd (2019). "Limited dispersion and quick degradation of environmental DNA in fish ponds inferred by metabarcoding". Environmental DNA 1 (3): 238–250. doi:10.1002/edn3.24.
- ↑ Salter, Ian (2018). "Seasonal variability in the persistence of dissolved environmental DNA (EDNA) in a marine system: The role of microbial nutrient limitation". PLOS ONE 13 (2): e0192409. doi:10.1371/journal.pone.0192409. PMID 29474423. Bibcode: 2018PLoSO..1392409S.
- ↑ Buxton, Andrew S.; Groombridge, Jim J.; Griffiths, Richard A. (2018). "Seasonal variation in environmental DNA detection in sediment and water samples". PLOS ONE 13 (1): e0191737. doi:10.1371/journal.pone.0191737. PMID 29352294. Bibcode: 2018PLoSO..1391737B.
- ↑ Collins, Rupert A.; Wangensteen, Owen S.; o'Gorman, Eoin J.; Mariani, Stefano; Sims, David W.; Genner, Martin J. (2018). "Persistence of environmental DNA in marine systems". Communications Biology 1: 185. doi:10.1038/s42003-018-0192-6. PMID 30417122.
- ↑ 143.0 143.1 Andruszkiewicz, Elizabeth A.; Koseff, Jeffrey R.; Fringer, Oliver B.; Ouellette, Nicholas T.; Lowe, Anna B.; Edwards, Christopher A.; Boehm, Alexandria B. (2019). "Modeling Environmental DNA Transport in the Coastal Ocean Using Lagrangian Particle Tracking". Frontiers in Marine Science 6. doi:10.3389/fmars.2019.00477.
- ↑ Eichmiller, Jessica J.; Bajer, Przemyslaw G.; Sorensen, Peter W. (2014). "The Relationship between the Distribution of Common Carp and Their Environmental DNA in a Small Lake". PLOS ONE 9 (11): e112611. doi:10.1371/journal.pone.0112611. PMID 25383965. Bibcode: 2014PLoSO...9k2611E.
- ↑ Gargan, Laura M.; Morato, Telmo; Pham, Christopher K.; Finarelli, John A.; Carlsson, Jeanette E. L.; Carlsson, Jens (2017). "Development of a sensitive detection method to survey pelagic biodiversity using eDNA and quantitative PCR: A case study of devil ray at seamounts". Marine Biology 164 (5): 112. doi:10.1007/s00227-017-3141-x. Bibcode: 2017MarBi.164..112G. https://zenodo.org/record/1213273.
- ↑ Yamamoto, Satoshi; Minami, Kenji; Fukaya, Keiichi; Takahashi, Kohji; Sawada, Hideki; Murakami, Hiroaki; Tsuji, Satsuki; Hashizume, Hiroki et al. (2016). "Environmental DNA as a 'Snapshot' of Fish Distribution: A Case Study of Japanese Jack Mackerel in Maizuru Bay, Sea of Japan". PLOS ONE 11 (3): e0149786. doi:10.1371/journal.pone.0149786. PMID 26933889. Bibcode: 2016PLoSO..1149786Y.
- ↑ 147.0 147.1 Knudsen, Steen Wilhelm; Ebert, Rasmus Bach; Hesselsøe, Martin; Kuntke, Franziska; Hassingboe, Jakob; Mortensen, Peter Bondgaard; Thomsen, Philip Francis; Sigsgaard, Eva Egelyng et al. (2019). "Species-specific detection and quantification of environmental DNA from marine fishes in the Baltic Sea". Journal of Experimental Marine Biology and Ecology 510: 31–45. doi:10.1016/j.jembe.2018.09.004.
- ↑ 148.0 148.1 148.2 148.3 148.4 148.5 Corinaldesi, C.; Tangherlini, M.; Manea, E.; Dell'Anno, A. (2018). "Extracellular DNA as a genetic recorder of microbial diversity in benthic deep-sea ecosystems". Scientific Reports 8 (1): 1839. doi:10.1038/s41598-018-20302-7. PMID 29382896. Bibcode: 2018NatSR...8.1839C.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 149.0 149.1 Dell'Anno, A.; Danovaro, R. (2005). "Extracellular DNA Plays a Key Role in Deep-Sea Ecosystem Functioning". Science 309 (5744): 2179. doi:10.1126/science.1117475. PMID 16195451.
- ↑ Corinaldesi, Cinzia; Dell'Anno, Antonio; Danovaro, Roberto (2007). "Viral infection plays a key role in extracellular DNA dynamics in marine anoxic systems". Limnology and Oceanography 52 (2): 508–516. doi:10.4319/lo.2007.52.2.0508. Bibcode: 2007LimOc..52..508C.
- ↑ Dell'Anno, Antonio; Corinaldesi, Cinzia; Danovaro, Roberto (2015). "Virus decomposition provides an important contribution to benthic deep-sea ecosystem functioning". Proceedings of the National Academy of Sciences 112 (16): E2014–E2019. doi:10.1073/pnas.1422234112. PMID 25848024. Bibcode: 2015PNAS..112E2014D.
- ↑ Nielsen, Kaare M.; Johnsen, Pål J.; Bensasson, Douda; Daffonchio, Daniele (2007). "Release and persistence of extracellular DNA in the environment". Environmental Biosafety Research 6 (1–2): 37–53. doi:10.1051/ebr:2007031. PMID 17961479.
- ↑ Coolen, Marco J. L.; Overmann, Jörg (1998). "Analysis of Subfossil Molecular Remains of Purple Sulfur Bacteria in a Lake Sediment". Applied and Environmental Microbiology 64 (11): 4513–4521. doi:10.1128/AEM.64.11.4513-4521.1998. PMID 9797316. Bibcode: 1998ApEnM..64.4513C.
- ↑ Coolen, M.; Muyzer, Gerard; Rijpstra, W. Irene C.; Schouten, Stefan; Volkman, John K.; Sinninghe Damsté, Jaap S. (2004). "Combined DNA and lipid analyses of sediments reveal changes in Holocene haptophyte and diatom populations in an Antarctic lake". Earth and Planetary Science Letters 223 (1–2): 225–239. doi:10.1016/j.epsl.2004.04.014. Bibcode: 2004E&PSL.223..225C.
- ↑ 155.0 155.1 Coolen, M. J. L.; Orsi, W. D.; Balkema, C.; Quince, C.; Harris, K.; Sylva, S. P.; Filipova-Marinova, M.; Giosan, L. (2013). "Evolution of the plankton paleome in the Black Sea from the Deglacial to Anthropocene". Proceedings of the National Academy of Sciences 110 (21): 8609–8614. doi:10.1073/pnas.1219283110. PMID 23650351. Bibcode: 2013PNAS..110.8609C.
- ↑ 156.0 156.1 Corinaldesi, C.; Tangherlini, M.; Luna, G. M.; Dell'Anno, A. (2014). "Extracellular DNA can preserve the genetic signatures of present and past viral infection events in deep hypersaline anoxic basins". Proceedings of the Royal Society B: Biological Sciences 281 (1780). doi:10.1098/rspb.2013.3299. PMID 24523277.
- ↑ 157.0 157.1 Boere, Arjan C.; Rijpstra, W. Irene C.; De Lange, Gert J.; Malinverno, Elisa; Sinninghe Damsté, Jaap S.; Coolen, Marco J. L. (2011). "Exploring preserved fossil dinoflagellate and haptophyte DNA signatures to infer ecological and environmental changes during deposition of sapropel S1 in the eastern Mediterranean". Paleoceanography 26 (2): n/a. doi:10.1029/2010PA001948. Bibcode: 2011PalOc..26.2204B. http://www.vliz.be/imisdocs/publications/71/255171.pdf.
- ↑ Coolen, M. J. L.; Cypionka, H.; Sass, A. M.; Sass, H.; Overmann, J. (2002). "Ongoing Modification of Mediterranean Pleistocene Sapropels Mediated by Prokaryotes". Science 296 (5577): 2407–2410. doi:10.1126/science.1071893. PMID 12089447. Bibcode: 2002Sci...296.2407C.
- ↑ Coolen, Marco J. L.; Overmann, Jörg (2007). "217 000-year-old DNA sequences of green sulfur bacteria in Mediterranean sapropels and their implications for the reconstruction of the paleoenvironment". Environmental Microbiology 9 (1): 238–249. doi:10.1111/j.1462-2920.2006.01134.x. PMID 17227428.
- ↑ Coolen, Marco J. L.; Volkman, John K.; Abbas, Ben; Muyzer, Gerard; Schouten, Stefan; Sinninghe Damsté, Jaap S. (2007). "Identification of organic matter sources in sulfidic late Holocene Antarctic fjord sediments from fossil rDNA sequence analysis". Paleoceanography 22 (2): PA2211. doi:10.1029/2006PA001309. Bibcode: 2007PalOc..22.2211C.
- ↑ Coolen, Marco J.L.; Saenz, James P.; Giosan, Liviu; Trowbridge, Nan Y.; Dimitrov, Petko; Dimitrov, Dimitar; Eglinton, Timothy I. (2009). "DNA and lipid molecular stratigraphic records of haptophyte succession in the Black Sea during the Holocene". Earth and Planetary Science Letters 284 (3–4): 610–621. doi:10.1016/j.epsl.2009.05.029. Bibcode: 2009E&PSL.284..610C.
- ↑ Dell'Anno, Antonio; Corinaldesi, Cinzia; Stavrakakis, Spyros; Lykousis, Vasilis; Danovaro, Roberto (2005). "Pelagic-Benthic Coupling and Diagenesis of Nucleic Acids in a Deep-Sea Continental Margin and an Open-Slope System of the Eastern Mediterranean". Applied and Environmental Microbiology 71 (10): 6070–6076. doi:10.1128/AEM.71.10.6070-6076.2005. PMID 16204523. Bibcode: 2005ApEnM..71.6070D.
- ↑ Levin, Lisa A.; Liu, Kon-Kee; Emeis, Kay-Christian; Breitburg, Denise L.; Cloern, James; Deutsch, Curtis; Giani, Michele; Goffart, Anne et al. (2015). "Comparative biogeochemistry–ecosystem–human interactions on dynamic continental margins". Journal of Marine Systems 141: 3–17. doi:10.1016/j.jmarsys.2014.04.016. Bibcode: 2015JMS...141....3L. https://digitalcommons.uri.edu/cgi/viewcontent.cgi?article=1630&context=gsofacpubs.
- ↑ Jørgensen, Bo Barker; Boetius, Antje (2007). "Feast and famine — microbial life in the deep-sea bed". Nature Reviews Microbiology 5 (10): 770–781. doi:10.1038/nrmicro1745. PMID 17828281.
- ↑ Orcutt, B. N.; Sylvan, J. B.; Knab, N. J.; Edwards, K. J. (2011). "Microbial Ecology of the Dark Ocean above, at, and below the Seafloor". Microbiology and Molecular Biology Reviews 75 (2): 361–422. doi:10.1128/MMBR.00039-10. PMID 21646433.
- ↑ Corinaldesi, Cinzia (2015). "New perspectives in benthic deep-sea microbial ecology". Frontiers in Marine Science 2. doi:10.3389/fmars.2015.00017.
- ↑ Zinger, Lucie; Amaral-Zettler, Linda A.; Fuhrman, Jed A.; Horner-Devine, M. Claire; Huse, Susan M.; Welch, David B. Mark; Martiny, Jennifer B. H.; Sogin, Mitchell et al. (2011). "Global Patterns of Bacterial Beta-Diversity in Seafloor and Seawater Ecosystems". PLOS ONE 6 (9): e24570. doi:10.1371/journal.pone.0024570. PMID 21931760. Bibcode: 2011PLoSO...624570Z.
- ↑ Bienhold, Christina; Boetius, Antje; Ramette, Alban (2012). "The energy–diversity relationship of complex bacterial communities in Arctic deep-sea sediments". The ISME Journal 6 (4): 724–732. doi:10.1038/ismej.2011.140. PMID 22071347.
- ↑ Zinger, L.; Boetius, A.; Ramette, A. (2014). "Bacterial taxa–area and distance–decay relationships in marine environments". Molecular Ecology 23 (4): 954–964. doi:10.1111/mec.12640. PMID 24460915. Bibcode: 2014MolEc..23..954Z.
- ↑ Drummond, Alexei J.; Newcomb, Richard D.; Buckley, Thomas R.; Xie, Dong; Dopheide, Andrew; Potter, Benjamin CM; Heled, Joseph; Ross, Howard A. et al. (2015). "Evaluating a multigene environmental DNA approach for biodiversity assessment". GigaScience 4: 46. doi:10.1186/s13742-015-0086-1. PMID 26445670.
- ↑ Corinaldesi, C.; Beolchini, F.; Dell'Anno, A. (2008). "Damage and degradation rates of extracellular DNA in marine sediments: Implications for the preservation of gene sequences". Molecular Ecology 17 (17): 3939–3951. doi:10.1111/j.1365-294X.2008.03880.x. PMID 18643876. Bibcode: 2008MolEc..17.3939C.
- ↑ Pedersen, Mikkel Winther; Overballe-Petersen, Søren; Ermini, Luca; Sarkissian, Clio Der; Haile, James; Hellstrom, Micaela; Spens, Johan; Thomsen, Philip Francis et al. (2015). "Ancient and modern environmental DNA". Philosophical Transactions of the Royal Society B: Biological Sciences 370 (1660). doi:10.1098/rstb.2013.0383. PMID 25487334.
- ↑ Parducci, Laura; Bennett, Keith D.; Ficetola, Gentile Francesco; Alsos, Inger Greve; Suyama, Yoshihisa; Wood, Jamie R.; Pedersen, Mikkel Winther (2017). "Ancient plant DNA in lake sediments". New Phytologist 214 (3): 924–942. doi:10.1111/nph.14470. PMID 28370025.
- ↑ Armbrecht, Linda H.; Coolen, Marco J.L.; Lejzerowicz, Franck; George, Simon C.; Negandhi, Karita; Suzuki, Yohey; Young, Jennifer; Foster, Nicole R. et al. (2019). "Ancient DNA from marine sediments: Precautions and considerations for seafloor coring, sample handling and data generation". Earth-Science Reviews 196: 102887. doi:10.1016/j.earscirev.2019.102887. Bibcode: 2019ESRv..19602887A.
- ↑ De Vargas, C. et al. (2015). "Eukaryotic plankton diversity in the sunlit ocean". Science 348 (6237). doi:10.1126/science.1261605. PMID 25999516. https://archimer.ifremer.fr/doc/00270/38135/.
- ↑ Iversen, M. H.; Ploug, H. (2010). "Ballast minerals and the sinking carbon flux in the ocean: Carbon-specific respiration rates and sinking velocity of marine snow aggregates". Biogeosciences 7 (9): 2613–2624. doi:10.5194/bg-7-2613-2010. Bibcode: 2010BGeo....7.2613I.
- ↑ Takahashi, Kozo; Be, Allan W.H. (1984). "Planktonic foraminifera: Factors controlling sinking speeds". Deep Sea Research Part A. Oceanographic Research Papers 31 (12): 1477–1500. doi:10.1016/0198-0149(84)90083-9. Bibcode: 1984DSRA...31.1477T.
- ↑ 178.0 178.1 De Schepper, Stijn; Ray, Jessica L.; Skaar, Katrine Sandnes; Sadatzki, Henrik; Ijaz, Umer Z.; Stein, Ruediger; Larsen, Aud (2019). "The potential of sedimentary ancient DNA for reconstructing past sea ice evolution". The ISME Journal 13 (10): 2566–2577. doi:10.1038/s41396-019-0457-1. PMID 31235841.
- ↑ 179.0 179.1 179.2 Barrenechea Angeles, Inès; Lejzerowicz, Franck; Cordier, Tristan; Scheplitz, Janin; Kucera, Michal; Ariztegui, Daniel; Pawlowski, Jan; Morard, Raphaël (2020). "Planktonic foraminifera eDNA signature deposited on the seafloor remains preserved after burial in marine sediments". Scientific Reports 10 (1): 20351. doi:10.1038/s41598-020-77179-8. PMID 33230106.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 180.0 180.1 Briggs, Lisa (2020). "Ancient DNA Research in Maritime and Underwater Archaeology: Pitfalls, Promise, and Future Directions". Open Quaternary 6. doi:10.5334/oq.71.
- ↑ 181.0 181.1 Morard, Raphaël; Lejzerowicz, Franck; Darling, Kate F.; Lecroq-Bennet, Béatrice; Winther Pedersen, Mikkel; Orlando, Ludovic; Pawlowski, Jan; Mulitza, Stefan et al. (2017). "Planktonic foraminifera-derived environmental DNA extracted from abyssal sediments preserves patterns of plankton macroecology". Biogeosciences 14 (11): 2741–2754. doi:10.5194/bg-14-2741-2017. Bibcode: 2017BGeo...14.2741M.
- ↑ 182.0 182.1 Corinaldesi, C.; Beolchini, F.; Dell'Anno, A. (2008). "Damage and degradation rates of extracellular DNA in marine sediments: Implications for the preservation of gene sequences". Molecular Ecology 17 (17): 3939–3951. doi:10.1111/j.1365-294X.2008.03880.x. PMID 18643876. Bibcode: 2008MolEc..17.3939C.
- ↑ 183.0 183.1 Corinaldesi, Cinzia; Dell'Anno, Antonio; Danovaro, Roberto (2007). "Early diagenesis and trophic role of extracellular DNA in different benthic ecosystems". Limnology and Oceanography 52 (4): 1710–1717. doi:10.4319/lo.2007.52.4.1710. Bibcode: 2007LimOc..52.1710C.
- ↑ 184.0 184.1 Corinaldesi, C.; Barucca, M.; Luna, G. M.; Dell'Anno, A. (2011). "Preservation, origin and genetic imprint of extracellular DNA in permanently anoxic deep-sea sediments". Molecular Ecology 20 (3): 642–654. doi:10.1111/j.1365-294X.2010.04958.x. PMID 21155913. Bibcode: 2011MolEc..20..642C.
- ↑ Guillou, Laure et al. (2012). "The Protist Ribosomal Reference database (PR2): A catalog of unicellular eukaryote Small Sub-Unit rRNA sequences with curated taxonomy". Nucleic Acids Research 41 (Database issue): D597–D604. doi:10.1093/nar/gks1160. PMID 23193267.
- ↑ Schiebel, Ralf; Hemleben, Christoph (2017-02-17) (in en). Planktic Foraminifers in the Modern Ocean. Springer. ISBN 978-3-662-50297-6. https://books.google.com/books?id=o8omDgAAQBAJ&q=%22Planktic+Foraminifers+in+the+Modern+Ocean%22.
- ↑ Morard, Raphaël; Darling, Kate F.; Mahé, Frédéric; Audic, Stéphane; Ujiié, Yurika; Weiner, Agnes K. M.; André, Aurore; Seears, Heidi A. et al. (2015). "PFR2: A curated database of planktonic foraminifera 18S ribosomal DNA as a resource for studies of plankton ecology, biogeography and evolution". Molecular Ecology Resources 15 (6): 1472–1485. doi:10.1111/1755-0998.12410. PMID 25828689. https://hal.sorbonne-universite.fr/hal-01149023v2/file/2015_morard.pdf.
- ↑ Morard, Raphaël; Vollmar, Nele M.; Greco, Mattia; Kucera, Michal (2019). "Unassigned diversity of planktonic foraminifera from environmental sequencing revealed as known but neglected species". PLOS ONE 14 (3): e0213936. doi:10.1371/journal.pone.0213936. PMID 30897140. Bibcode: 2019PLoSO..1413936M.
- ↑ Rutherford, Scott; d'Hondt, Steven; Prell, Warren (1999). "Environmental controls on the geographic distribution of zooplankton diversity". Nature 400 (6746): 749–753. doi:10.1038/23449. Bibcode: 1999Natur.400..749R.
- ↑ Siccha, Michael; Kucera, Michal (2017). "ForCenS, a curated database of planktonic foraminifera census counts in marine surface sediment samples". Scientific Data 4: 170109. doi:10.1038/sdata.2017.109. PMID 28829434. Bibcode: 2017NatSD...470109S.
- ↑ Lejzerowicz, Franck; Esling, Philippe; Majewski, Wojciech; Szczuciński, Witold; Decelle, Johan; Obadia, Cyril; Arbizu, Pedro Martinez; Pawlowski, Jan (2013). "Ancient DNA complements microfossil record in deep-sea subsurface sediments". Biology Letters 9 (4). doi:10.1098/rsbl.2013.0283. PMID 23658006.
- ↑ Pawłowska, J.; Lejzerowicz, F.; Esling, P.; Szczuciński, W.; Zajączkowski, M.; Pawlowski, J. (2014). "Ancient DNA sheds new light on the Svalbard foraminiferal fossil record of the last millennium". Geobiology 12 (4): 277–288. doi:10.1111/gbi.12087. PMID 24730667. Bibcode: 2014Gbio...12..277P.
- ↑ Pawłowska, Joanna; Zajączkowski, Marek; Łącka, Magdalena; Lejzerowicz, Franck; Esling, Philippe; Pawlowski, Jan (2016). "Palaeoceanographic changes in Hornsund Fjord (Spitsbergen, Svalbard) over the last millennium: New insights from ancient DNA". Climate of the Past 12 (7): 1459–1472. doi:10.5194/cp-12-1459-2016. Bibcode: 2016CliPa..12.1459P.
- ↑ Szczuciński, Witold; Pawłowska, Joanna; Lejzerowicz, Franck; Nishimura, Yuichi; Kokociński, Mikołaj; Majewski, Wojciech; Nakamura, Yugo; Pawlowski, Jan (2016). "Ancient sedimentary DNA reveals past tsunami deposits". Marine Geology 381: 29–33. doi:10.1016/j.margeo.2016.08.006. Bibcode: 2016MGeol.381...29S.
- ↑ Nunn, Jack (2020). "Science for All - Publicly funded research report (June 2018-December 2019)" (in en-US). Figshare. doi:10.26181/5eba630a64e08. https://doi.org/10.26181/5eba630a64e08.
Further references
- Schallenberg, Lena; Wood, Susie A.; Pochon, Xavier; Pearman, John K. (2020). "What Can DNA in the Environment Tell Us About an Ecosystem?". Frontiers for Young Minds 7. doi:10.3389/frym.2019.00150.
External links
 |
![Global ecosystem and biodiversity monitoring with environmental DNA metabarcoding [6]](/wiki/images/thumb/f/f7/Global_ecosystem_and_biodiversity_monitoring_with_environmental_DNA_metabarcoding.jpg/622px-Global_ecosystem_and_biodiversity_monitoring_with_environmental_DNA_metabarcoding.jpg)
![Applications of environmental DNA metabarcoding in aquatic and terrestrial ecosystems [6]](/wiki/images/thumb/2/28/Applications_of_environmental_DNA_metabarcoding_in_aquatic_and_terrestrial_ecosystems.jpg/527px-Applications_of_environmental_DNA_metabarcoding_in_aquatic_and_terrestrial_ecosystems.jpg)
![Relic DNA dynamics [39]](/wiki/images/thumb/e/e4/Modeling_relic_DNA_dynamics.jpg/534px-Modeling_relic_DNA_dynamics.jpg)
![(a) Metabarcoding is the amplification and analysis of equally sized DNA fragments from a total DNA extract. (b) Metagenomics is the extraction, amplification, and analysis of all DNA fragments independent of size. (c) Target-capture describes the enrichment and analysis of specific (chosen) DNA fragments independent of size from a total DNA extract.[50]](/wiki/images/thumb/1/15/Methodological_approaches_in_modern_and_ancient_marine_genomics.jpg/592px-Methodological_approaches_in_modern_and_ancient_marine_genomics.jpg)
![Drilling vessel recovering a sediment core for sedaDNA analysis and hypothetical past marine community composition [50]](/wiki/images/thumb/3/3a/Drilling_vessel_recovering_a_sediment_core_for_sedaDNA_analysis.jpg/379px-Drilling_vessel_recovering_a_sediment_core_for_sedaDNA_analysis.jpg)
![Subglacial aquatic sediment continuous coring [56]](/wiki/images/thumb/f/f5/Subglacial_aquatic_sediment_continuous_coring.jpg/189px-Subglacial_aquatic_sediment_continuous_coring.jpg)
![Bipartite plot for the COI gene, showing from which plants each arthropod family is obtained. Plant names: Angeli (Angelica archangelica), Centau (Centaurea jacea), Daucus (Daucus carota), Echium (Echium vulgare), Eupato (Eupatorium cannabinum), Solida (Solidago canadensis), Tanace (Tanacetum vulgare).[1]](/wiki/images/thumb/1/10/Differentiation_of_arthropod_communities_by_plant_species.webp/621px-Differentiation_of_arthropod_communities_by_plant_species.webp.png)


![OTU (operational taxonomic unit) network of the extracellular DNA pools from the sediments of the different continental margins.[148]](/wiki/images/thumb/a/ac/Extracellular_DNA_pools_from_continental_margin_sediments.webp/465px-Extracellular_DNA_pools_from_continental_margin_sediments.webp.png)
